In the evolving battleground of oncology, few molecular targets have captured as much attention and complexity as the MYC protein. Renowned as a master regulator within the cellular environment, MYC orchestrates a myriad of biological pathways essential to both normal physiology and malignant transformation. A recent comprehensive review published in Genes & Diseases delves deeply into the multifaceted role of MYC in cancer biology and unveils cutting-edge therapeutic strategies aimed at exploiting this elusive yet critical oncogene.
MYC functions as a pivotal transcription factor that governs key aspects of cell proliferation, metabolism, and survival. Its dysregulation is implicated in roughly 70% of human cancers, underscoring its broad impact across diverse tumor types. The protein’s ability to influence cell cycle progression, apoptosis resistance, angiogenesis promotion, and immune evasion renders it a formidable driver of tumor aggressiveness. Furthermore, MYC’s involvement in mediating resistance to a variety of chemotherapeutic agents elevates its status from a mere oncogene to a significant barrier to effective treatment outcomes.
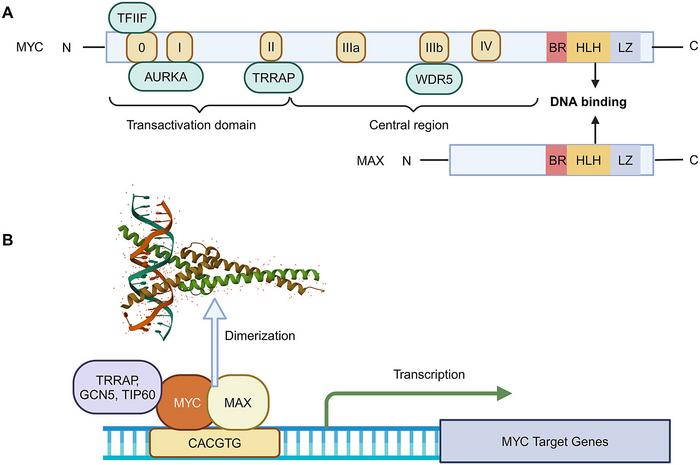
Despite its central oncogenicity, MYC has historically been labeled “undruggable.” Its intrinsically disordered structure lacks the defined pockets typically targeted by traditional small molecule drugs, and MYC’s extensive interactions across protein networks complicate direct inhibition. However, the tide is turning. Recent advancements propose interfering with the critical MYC-MAX heterodimerization, a process essential for MYC’s transcriptional activity. Disrupting this dimer formation effectively silences MYC-driven gene expression, providing a tangible therapeutic avenue.
Significant progress has been made through compounds such as OMO-103, a molecule engineered to selectively disrupt the MYC-MAX interface. Early-phase clinical evaluations reveal that OMO-103 can impede tumor proliferation by dismantling oncogenic transcription programs, signaling a breakthrough in MYC-targeted therapy. This direct blockade signifies a paradigm shift, demonstrating that with precision drug design, even proteins once considered refractory to intervention can be harnessed therapeutically.
In tandem with direct inhibition, the landscape of MYC targeting expands towards indirect strategies. One promising route involves the suppression of MYC at the transcriptional or translational level. By attenuating MYC mRNA synthesis or destabilizing its transcripts, it is possible to reduce the protein’s cellular abundance. These approaches often utilize antisense oligonucleotides, RNA interference technologies, or small molecules that interfere with transcriptional regulators upstream of MYC. Such tactics delicately balance efficacy with reduced off-target toxicity.
Moreover, promoting the degradation of existing MYC protein pools emerges as another compelling strategy. Novel proteolysis-targeting chimeras (PROTACs) exploit the cell’s inherent ubiquitin-proteasome system to tag MYC for destruction. This method executes the selective clearance of MYC without inhibiting its function directly, diversifying the arsenal against tumors addicted to this oncoprotein. PROTAC technology heralds an era where targeted protein elimination can surmount obstacles imposed by structural disarray in challenging targets like MYC.
Another layer of innovation rests in synthetic lethality approaches, designed to exploit cellular dependencies unique to MYC-overexpressing cancer cells. By identifying pathways indispensable to the survival of tumors driven by elevated MYC, researchers can deploy drugs that selectively disable these auxiliary systems, sparing normal cells that lack such reliance. This precision approach carries immense potential for minimizing collateral damage, a long-standing problem in conventional chemotherapy.
The integration of advanced small molecule inhibitors with protein degradation technologies sets the stage for combination therapies. Such regimens seek to enhance therapeutic efficacy through synergistic mechanisms, potentially overcoming monotherapy resistance that commonly hampers clinical success. Precision medicine principles guide these combinations, tailoring treatment to the tumor’s MYC expression profile and molecular context, thereby maximizing patient benefit while mitigating adverse effects.
However, the intricate biology of MYC necessitates careful consideration of context-dependent effects. MYC’s influence extends beyond tumor cells, participating in normal tissue regeneration and maintenance. Broad-spectrum or indiscriminate MYC inhibition risks impairing physiological processes, potentially leading to premature aging phenotypes or compromised tissue homeostasis. Consequently, therapeutic windows must be meticulously defined, and biomarkers of MYC activity must inform patient selection.
Advancements in molecular understanding have illuminated MYC’s extensive network of interacting partners, including transcriptional cofactors, chromatin remodelers, and signaling intermediaries. These insights allow for novel opportunities to modulate MYC’s oncogenic output indirectly by targeting critical nodes within its regulatory circuitry. Combination targeting of MYC and its ancillary pathways may reduce compensatory mechanisms that lead to therapeutic resistance, heralding more durable clinical responses.
The critical examination of MYC’s role in immune evasion also opens avenues for integrating MYC-targeted therapy with immuno-oncology. MYC’s suppression of immune surveillance mechanisms fosters an immunosuppressive tumor microenvironment. Disrupting MYC signaling could restore immune recognition and augment responses to checkpoint inhibitors or cellular immunotherapies. Such interdisciplinary treatments embody the modern holistic approach necessary to confront complex cancer biology.
In summary, the evolving narrative of MYC as an oncogenic driver and therapeutic target reveals a transition from “undruggable” enigma to an actionable gateway. The convergence of structural biology, chemical innovation, molecular genetics, and clinical research has expedited the emergence of multifaceted therapeutic modalities that directly or indirectly attenuate MYC function. This multidisciplinary momentum not only redefines the therapeutic landscape for MYC-driven cancers but also exemplifies the power of precision medicine to conquer historically intractable biological challenges.
As investigators continue to unravel the nuances of MYC regulation and exploit its vulnerabilities, the future promises novel and effective cancer treatment strategies. The story of MYC underscores the broader scientific journey from understanding fundamental oncogenic processes to translating that knowledge into transformative patient outcomes. With ongoing clinical trials and burgeoning drug development pipelines, MYC-directed therapies stand at the vanguard of oncology innovation, poised to reshape cancer care paradigms worldwide.
Subject of Research: MYC protein regulation and therapeutic targeting in oncology
Article Title: Targeting MYC: Multidimensional regulation and therapeutic strategies in oncology
Web References:
DOI – http://dx.doi.org/10.1016/j.gendis.2024.101435
References:
Yingying Duan, Zhaoshuo Liu, Qilin Wang, Junyou Zhang, Jiaxin Liu, Ziyi Zhang, Chunyan Li, Targeting MYC: Multidimensional regulation and therapeutic strategies in oncology, Genes & Diseases, Volume 12, Issue 4, 2025, 101435
Image Credits: Genes & Diseases
Keywords: MYC, oncogene, cancer therapy, protein degradation, PROTAC, MYC-MAX complex, synthetic lethality, small molecule inhibitors, transcription factor, drug resistance, precision medicine, immuno-oncology
Tags: angiogenesis and cancer survivalcancer treatment advancementschallenges in drugging MYCimmune evasion in tumor cellsinnovative cancer therapiesmolecular oncology researchMYC oncogene targeting strategiesMYC protein role in tumor biologyovercoming drug resistance in oncologypersonalized cancer treatment strategiestherapeutic approaches for MYC dysregulationtranscription factors in cancer