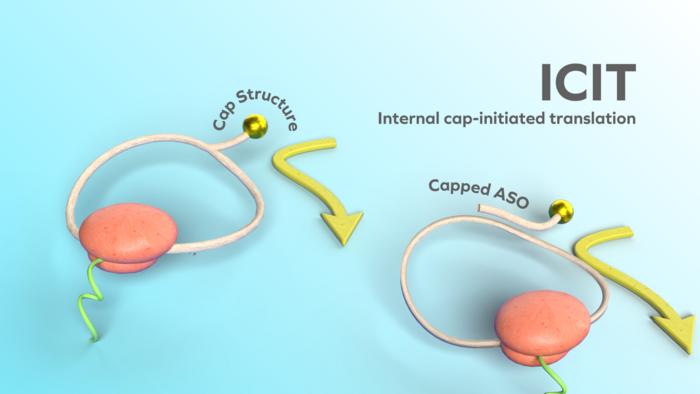
A groundbreaking study led by Hiroshi Abe and his research team at Nagoya University is poised to revolutionize the field of mRNA medicine, particularly in the treatment of cancer and protein disorders. The research, published in Nature Biotechnology, introduces a novel mechanism termed Internal Cap-Initiated Translation (ICIT), which promises to supercharge protein synthesis in targeted cells while leaving healthy cells untouched. This innovation could pave the way for precise, targeted therapies that significantly improve patient outcomes and minimize side effects.
The ICIT mechanism exploits the advantages of circular mRNA, a newer class of mRNA that has gained attention for its enhanced stability and diminished inflammatory responses when compared to traditional linear mRNA. Unlike linear mRNA, which is vulnerable to degradation due to its terminal structures, circular mRNA maintains its integrity, allowing for a prolonged and sustained protein translation process. However, a significant roadblock in utilizing circular mRNAs effectively has been their translation efficiency in biological contexts, which has historically lagged behind that of linear counterparts.
To address this challenge, Abe’s team made a pivotal advancement by integrating an internal cap structure directly into the circular mRNA. This innovation streamlines the process of translation initiation, eliminating the need for long internal ribosome entry sites (IRES) that are often inefficient and difficult to optimize. The introduction of this cap structure enhances the translation process, resulting in a remarkable boost in protein production, which could have far-reaching implications for therapeutic applications.
Among the various constructs tested, the Cap-circRNA variant exhibited an astonishing capability for synthesizing protein—up to 200 times more efficiently than traditional circular mRNAs incorporating IRES sequences. This newfound efficiency extends the lifespan of protein synthesis far beyond that of conventional mRNA formats, which tend to degrade more rapidly over time. This characteristic endows Cap-circRNA with unique properties, making it a strong candidate for developing precision therapies capable of addressing specific diseases.
One exciting aspect of this research lies in its potential applications in cancer treatment. The precision with which ICIT allows for regulated protein translation means that it can be tailored to target specific RNA markers prevalent in diseased cells. By selectively activating mRNA to produce therapeutic proteins specifically within tumor cells, this method not only enhances the effectiveness of treatment but also mitigates the adverse effects commonly associated with systemic therapies that impact healthy cells.
For instance, Abe’s research indicates that by leveraging the ICIT mechanism, it is possible to design mRNA constructs that target HULC lncRNA, an RNA biomarker significantly upregulated in liver cancer cells. This targeted approach resulted in a dramatic over 50-fold increase in the synthesis of therapeutic proteins when these cancer cells are present, underscoring the precision offered by this scientific breakthrough. This selectivity is critical in reducing the risk of inadvertent damage to normal cells, which is often a consequence of current cancer treatment modalities.
The implications of this innovative approach extend far beyond oncology. Abe emphasized the potential for this technology to transform various areas within mRNA medicine, including antibody therapies, genome editing, and therapies aimed at replacing dysfunctional proteins. As the current landscape of mRNA therapies often demands frequent injections due to the instability of the mRNA, the robust nature of Cap-circRNA could herald a new era where patients no longer face the burden of multiple treatments, thus improving overall quality of life for those affected by chronic conditions.
Abe’s research also suggests the presence of similar mechanisms of translation control occurring naturally in cellular environments, particularly through the interplay of long non-coding RNAs and mRNA constructs. By unraveling these intricate interactions, there lies the potential for developing entirely new therapeutic strategies aimed at a wide array of diseases, further extending the horizon of personalized medicine.
Moreover, the implications of ICIT echo in the broader narrative of developing targeted therapies. By deploying biomarkers inherent to diseased cells, researchers could potentially design mRNA constructs that would only activate under certain disease conditions, leading to the production of proteins that induce apoptosis or programmed cell death selectively in cancerous tissues. This revolutionary paradigm shift towards targeted, cell-specific therapies emphasizes the need for ongoing research into the capabilities of mRNA and its derivatives.
As researchers delve deeper into this promising field, Abe’s discoveries illuminate pathways not merely for treating diseases but for rethinking how therapies are conceptualized. The prospect of tailoring treatment strategies to individual patient needs signifies a dramatic departure from conventional one-size-fits-all approaches, positioning mRNA medicine at the forefront of the future of healthcare.
In summary, the pioneering work of Abe and his team signifies a leap towards making the dream of precision medicine in mRNA therapies a tangible reality. By harnessing the power of circular mRNA through the ICIT mechanism, the potential to innovate cancer treatments and address disorders stemming from irregular protein synthesis is now within reach, laying the foundation for a healthier tomorrow. As we stand on the brink of a new age in medicine, it is imperative to pay close attention to these advancements and their impact on future therapeutic strategies.
This breakthrough not only brings hope to the fields of oncology and protein disorders but also inspires innovative thinking about how we can design treatments that are more effective, efficient, and targeted than ever before. As the medical community embraces this shift towards precision therapy, it opens the door to new possibilities that could transform patient care for generations to come.
Subject of Research: Internal Cap-Initiated Translation (ICIT) mechanism in circular mRNA
Article Title: Internal cap-initiated translation for efficient protein production from circular mRNA
News Publication Date: 19-Feb-2025
Web References: Nature Biotechnology
References: Nature Biotechnology
Image Credits: Mizuki Tada
Keywords: mRNA translation, Protein synthesis, Drug targets, Gene targeting, Cell therapies, Liver cancer, Antibody therapy.
Tags: cancer treatment innovationscircular mRNA benefitsInternal Cap-Initiated Translation mechanismminimizing side effects in therapiesmRNA stability improvementsmRNA technology advancementsNagoya University research breakthroughsnovel translation initiation strategiesprecision medicine developmentsprotein disorder therapiesprotein synthesis enhancementtargeted gene therapies