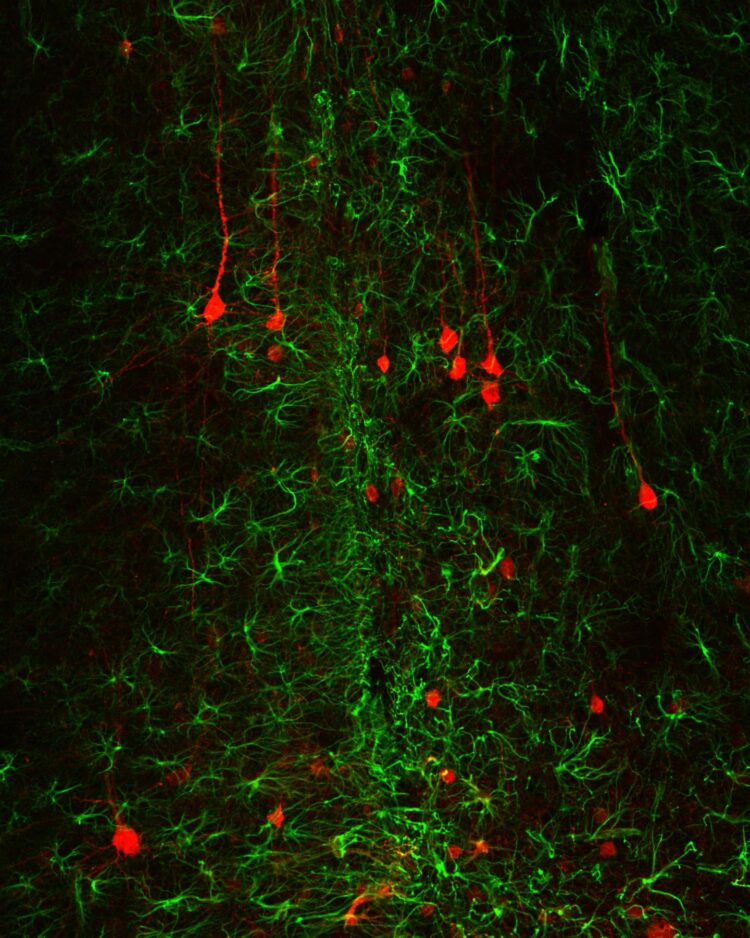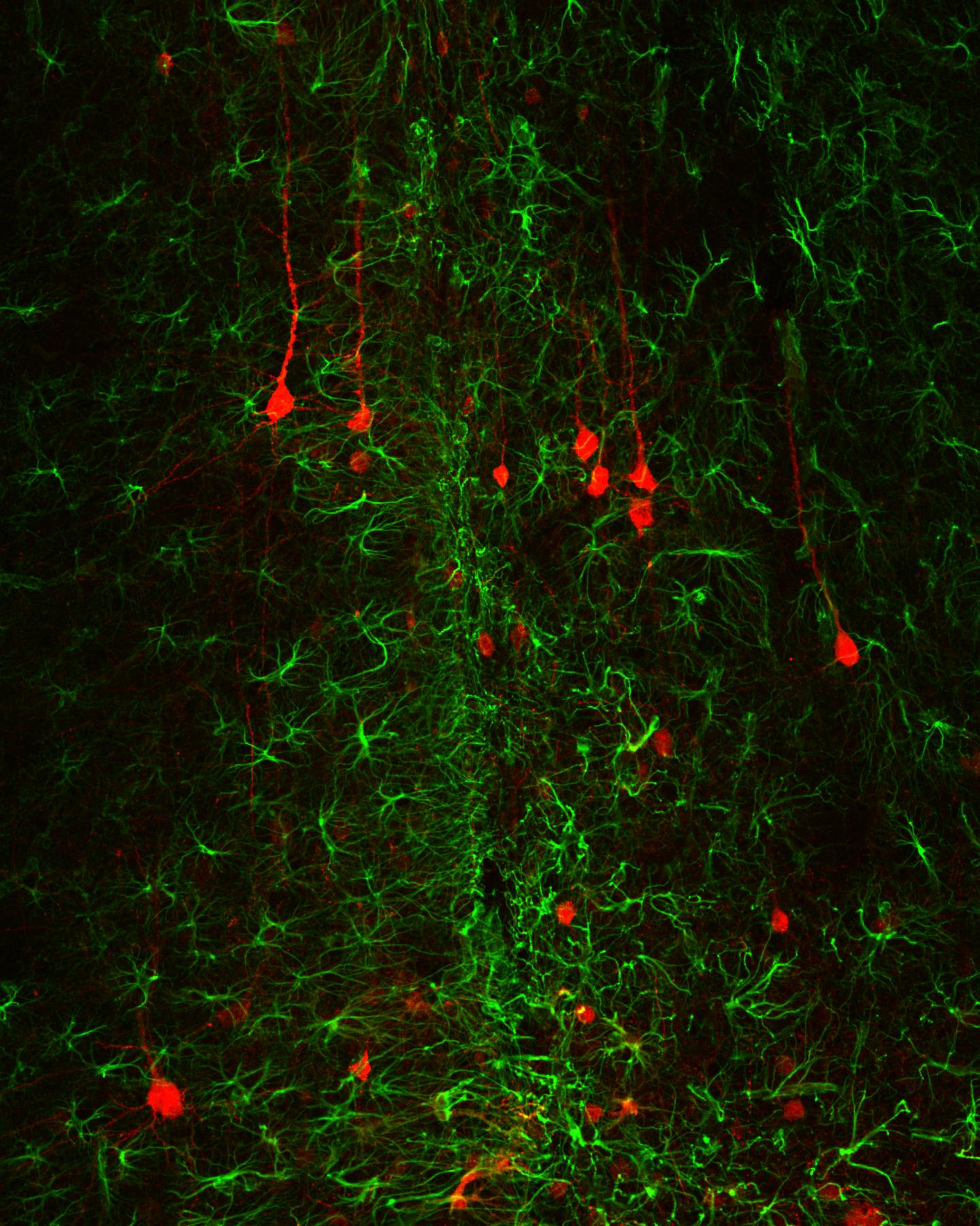
Credit: Jinan University
Brain or spinal cord injury often results in glial scar tissue that is correlated to neural functional loss. Glial scar is a well-known obstacle for neural regeneration due to its dense glial cell composition and lack of functional neurons. A research team led by Prof. Gong Chen at Jinan University, Guangzhou, China, published an article on November 5th, 2020 in the current issue of Frontiers in Cellular Neuroscience, demonstrating that glial scar tissue can be reversed back to neuronal tissue through NeuroD1-based neuroregenerative gene therapy.
While glial scar may have certain initial protective function, it also inhibits neuronal growth and functional recovery. Over the past decades or even centuries, doctors and scientists have tried to tackle glial scar tissue through surgical removal or molecular ablation but the dreams have dashed one after another. The difficulty lies in the fact that glial scar is a double-edge sword–on one hand it serves as a defense barrier to prevent the injury spreading to neighboring healthy tissue but on the other hand, it also inhibits the interactions between the inside scar tissue and outside healthy tissue, hampering functional recovery. Thus, it becomes such a dilemma that one wants to get rid of the glial scar tissue yet one cannot kill the glial cells inside the glial scar tissue.
Now, Prof. Chen and his team has designed a clever way to solve this century-old problem by turning glial cells inside the scar tissue directly into functional new neurons, essentially reversing glial scar tissue back to functional neural tissue. The key technology Chen and team invented is what he coined “neuroregenerative gene therapy”, which is a gene therapy that can regenerate functional new neurons in the nervous system in adult mammals. Chen’s team has recently published a series of articles demonstrating that such neuroregenerative gene therapy not only can regenerate functional new neurons from internal glial cells but also can promote brain functional recovery in mouse models of ischemic stroke and Huntington’s disease. Coincidently, on the same day of November 5th, 2020, Chen’s team also published the first non-human primate study showing that such neuroregenerative gene therapy can convert astrocytes, one subtype of glial cells in the brain and spinal cord, into neurons in adult monkey brains with ischemic stroke, making one step further toward future clinical applications.
“Our neuroregenerative gene therapy is to use AAV vector to deliver neural transcription factors such as NeuroD1 into glial cells in the injury site and directly convert reactive glial cells into functional new neurons. While delivering AAV vector to express a transgene falls into the category of gene therapy, the outcome is regeneration of new neurons, which is typically the result of cell therapy. Therefore, our neuroregenerative gene therapy can be viewed as a gene therapy-mediated cell therapy, and belongs to a more broad category of regenerative medicine”, explained by Prof. Chen about this unique technology.
“In this study, we delivered a neural transcription factor NeuroD1 into reactive astrocytes induced by brain injury and demonstrated that NeuroD1 expression reduced toxic reactive astrocytes and generated functional new neurons. Accompanying astrocyte-to-neuron conversion, the remaining astrocytes were found dividing and repopulated themselves. Unexpectedly, we also found that following conversion, neuroinflammation was reduced, new blood vessels emerged, and blood-brain-barrier was restored, leading to new neural tissue composed of new neurons, new astrocytes, and new blood vessels in the injury site”, said the first author Dr. Lei Zhang excitingly about her work.
“It is worth to mention that we designed two sets of experiments in this study to demonstrate that NeuroD1 not only can convert reactive astrocytes into neurons shortly after brain injury, but also can convert scar-forming astrocytes into neurons long after injury, suggesting that such in vivo astrocyte-to-neuron conversion approach may have broad time windows for therapeutic interventions”, added the co-first author Dr. Zhuo-Fan Lei.
“This in vivo astrocyte-to-neuron conversion technology is an one-stone-two-birds approach that not only generates new neurons but also reduces reactive glial cells in the injury areas, turning glial scar tissue back to neuron-enriched tissue, which should be an effective solution long-sought to solve the glial scar problem”, concluded Prof. Chen.
###
Besides Prof. Chen, Dr. Lei Zhang, and Dr. Zhuo-Fan Lei, other contributors to this work include Dr. Ziyuan Guo, Dr. Zifei Pei, Dr. Yuchen Chen, Fengyu Zhang, Alice Cai, Gabriel Mok, Grace Lee, Vishal Swaminathan, Fan Wang, and Yuting Bai. This work was mainly supported by Charles H. Smith Endowment Fund to Prof. Chen when he led the team at Penn State University.
Media Contact
Qingsong Wang
[email protected]
Related Journal Article
http://dx.