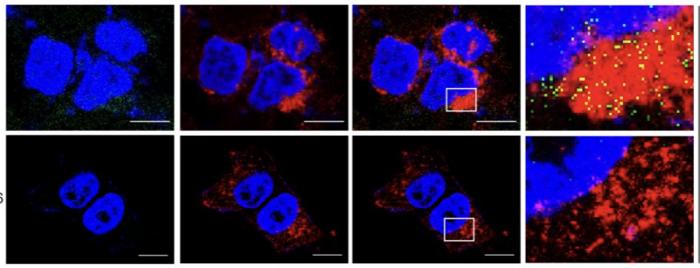
A groundbreaking advancement in cancer therapeutics promises to revolutionize the way large molecule drugs are delivered into cells, overcoming a longstanding obstacle in drug design. Researchers from Duke University School of Medicine, the University of Texas Health Science Center at San Antonio, and the University of Arkansas have unveiled a novel method that dramatically enhances the cellular uptake of proteolysis-targeting chimeras, commonly known as PROTACs. These large, complex molecules have historically struggled with poor bioavailability due to their size, limiting their therapeutic potential despite their promising capability to target and degrade disease-causing proteins.
By chemically engineering PROTAC molecules to actively engage the CD36 receptor, scientists have developed a strategy they term chemical endocytic medicinal chemistry (CEMC). This innovative approach turns the passive absorption paradigm on its head by harnessing the cell’s own uptake machinery to swallow large molecular drugs effectively. The results are striking: CD36-mediated delivery resulted in a 7.7 to 22.3-fold increase in intracellular drug concentration within cancer cells, leading to an up to 23-fold enhancement in therapeutic potency as demonstrated in rigorous preclinical studies published in the journal Cell.
The mouse model data reveal not only improved drug uptake but also a corresponding augmentation in tumor suppression without compromising the drug’s solubility or stability—two critical parameters for successful clinical application. This finding dispels previous concerns that enhancing cellular internalization might destabilize these large molecules or reduce their bioavailability. Instead, the CEMC strategy maintains these essential properties while ensuring that the active drug reaches its intracellular targets with unprecedented efficiency.
This advancement is particularly significant for the class of bRo5 molecules, which break the traditional ‘Rule of 5’ restrictions in medicinal chemistry and include various therapeutic agents beyond just PROTACs. PROTACs stand apart as unique targeted cancer therapies that work not by inhibiting enzymatic functions but by catalyzing the degradation of pathogenic proteins, effectively removing them from the cellular environment. This mode of action holds immense promise in combating drug resistance, a major challenge in oncology, as resistant tumors often evolve mechanisms to bypass enzymatic inhibitors but remain susceptible to protein degradation strategies.
The implications of CD36-mediated endocytosis extend well beyond oncology. While current attention is focused on PROTACs targeting cancer and neurodegenerative diseases like Parkinson’s disease, the underlying methodology could be universally applicable to many large, structurally complex therapeutic molecules previously deemed pharmacologically intractable. By opening the door to efficient intracellular delivery of these drugs, the study fosters new avenues for treating a variety of conditions that hinge on modulating protein expression or function inside cells.
Leading the charge to uncover CD36 as a key facilitator of drug internalization, Dr. Hong-yu Li from the University of Texas Health Science Center emphasized the paradigm shift heralded by this discovery. For decades, the scientific consensus held that molecules of this size could not cross membranes readily since the concept of endocytic uptake of chemically designed compounds was largely unexplored. The identification of CD36 as a receptor exploitable for drug entry has unlocked a previously inaccessible dimension of pharmacology, enabling researchers to optimize drug structures for receptor-mediated internalization rather than solely relying on membrane permeability.
The robustness of the findings is underscored by independent replication across the collaborative teams, including the work led by Dr. Zhiqiang Qin at the University of Arkansas Medical Sciences. Their collective research efforts confirm that this approach yields reproducible and significant improvements in drug performance, providing a strong foundation for the next phase of translational research and clinical development.
Beyond the mechanistic insight, this breakthrough also shifts the drug development focus from passive molecular optimization to harnessing intricate cellular processes. It paves the way for a future in which medicinal chemistry can systematically exploit receptor-mediated endocytosis, moving toward a more sophisticated and biologically integrated approach to drug design. Such strategies could herald a new era in which the molecular size and complexity of therapeutic agents are no longer insurmountable obstacles but deliberately tailored features to enhance receptor engagement and drug delivery.
Conventional cancer treatments, such as kinase inhibitors, target specific enzymatic activities within cancer cells but often fail to eliminate the proteins themselves, leaving residual functions that contribute to disease progression and therapeutic resistance. By contrast, PROTACs promote complete degradation of these proteins, thereby delivering a more comprehensive therapeutic effect and reducing the likelihood of resistance. By leveraging CD36 to increase the intracellular concentration of PROTAC drugs, this approach amplifies their ability to dismantle oncogenic proteins and impede tumor growth more effectively.
Eight oral PROTAC candidates are actively undergoing clinical trials, including a Phase 3 study evaluating one agent designed to degrade estrogen receptors in breast cancer. The ability to enhance PROTAC bioavailability through CD36 engagement could tremendously impact these ongoing trials and future drug pipelines, potentially increasing the success rates and broadening the applicability of protein-degrading therapies.
The multidisciplinary collaboration and generous support from major funding bodies such as the National Institutes of Health and the Cancer Prevention and Research Institute of Texas highlight the importance and high interest in developing next-generation treatment modalities. Together, these efforts signify a pivotal advancement with far-reaching consequences for medicinal chemistry, oncology, and beyond.
Ultimately, chemical endocytic medicinal chemistry stands as a beacon of innovation, propelling the drug discovery field toward overcoming size limitations and expanding the therapeutic toolbox with highly potent, large molecule drugs that can be efficiently internalized by cells. As research advances, this novel strategy holds the potential to not only improve patient outcomes in cancer but also transform treatment paradigms across various diseases driven by pathogenic proteins.
Subject of Research: Cells
Article Title: CD-36-mediated endocytosis of proteolysis-targeting chimeras
News Publication Date: April 17, 2025
Web References:
https://doi.org/10.1016/j.cell.2025.03.036
References:
Lin, H.-K., Li, H.-Y., Qin, Z., et al. “CD-36-mediated endocytosis of proteolysis-targeting chimeras,” Cell, April 17, 2025.
Image Credits:
Duke University School of Medicine
Keywords:
Cancer medication, Surface proteins, Drug design, Cancer cells, Drug resistance, Drug therapy
Tags: advancing cancer research technologiescancer drug delivery systemsCD36 protein role in drug deliverycellular uptake enhancement techniquesendocytosis in cancer therapeuticsengineering cell entry mechanismsinnovative cancer treatment strategieslarge molecule drug absorptionovercoming drug bioavailability challengesPROTACs drug developmentproteolysis-targeting chimerastraditional drug design limitations