A team of researchers from Hiroshima University has discovered a molecule that promotes the production of cancer cells. This molecule may prove to be a potential therapeutic target in the treatment of triple-negative breast cancer, an aggressive form of breast cancer.
Credit: Atsushi Saito, Hiroshima University
A team of researchers from Hiroshima University has discovered a molecule that promotes the production of cancer cells. This molecule may prove to be a potential therapeutic target in the treatment of triple-negative breast cancer, an aggressive form of breast cancer.
Their work was published in the journal Molecular Cancer Research on January 18, 2024.
Breast cancer is the most common type of cancer, ranking fifth among all cancers in cancer-related deaths. In 2020, there were 2.3 million new cases of breast cancers reported around the globe. In that year, breast cancer caused 685,000 deaths.
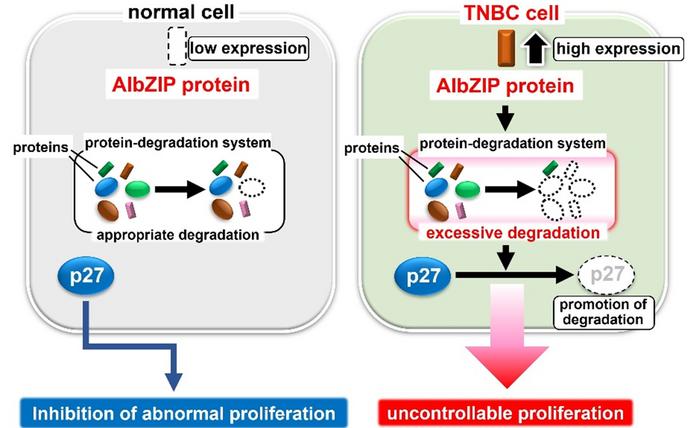
Several studies have reported that a molecule called AIbZIP (androgeninduced basic leucine zipper) promotes malignant behavior in different cancer types. So the research team examined the potential role of AIbZIP in malignant tumors. Their computer simulation analysis revealed that AIbZIP was highly expressed in the luminal androgen receptor subtype of triple negative breast cancer, playing a significant role in cell cycle regulation. They identified a novel mechanism by which AIbZIP regulates cancer cell proliferation in this type of breast cancer.
“We found that AIbZIP is highly upregulated in triple negative breast cancer. AIbZIP plays a crucial role for hyper proliferation of triple negative breast cancer cells by promoting the degradation of p27, a negative regulator for cell proliferation. Our study indicates that AIbZIP may be potential therapeutic target of triple negative breast cancer” said Atsushi Saito, an associate professor and Kazunori Imaizumi, a professor in the Department of Biochemistry, Graduate School of Biomedical and Health Sciences, Hiroshima University.
Breast cancer is divided into three types. These types, defined mainly by receptor and protein expression, are luminal breast cancer, HER2-positive breast cancer, and triple-negative breast cancer. Triple-negative breast cancer does not have the estrogen receptor, progesterone receptor, or HER2 (human epidermal growth factor receptor 2) found in the other two types of breast cancer. Without these receptors, or targets, this cancer is challenging to treat. “Among breast cancer, triple negative breast cancer has no known therapeutic targets and a poor prognosis. Therefore, new therapeutic targets and strategies against TNBC are required,” said Taichi Ito, a student in the Department of Biochemistry, Graduate School of Biomedical and Health Sciences, Hiroshima University.
Triple negative breast cancer has been further divided into subtypes, based on gene expression. The luminal androgen receptor is a subtype of triple negative breast cancer. This subtype represents 15 to 20 percent of all triple negative breast cancer cases.
Recent treatments for triple-negative breast cancer include chemotherapy, immunotherapy, and targeted therapies. These treatments hold promise, but they are not ideal solutions. Researchers continue to explore new therapeutic targets and strategies against triple-negative breast cancer.
The luminal androgen receptor subtype of triple negative breast cancer has a high expression of the androgen receptor and genes associated with androgenic hormonally-regulated pathways. As a result, researchers have made many attempts to develop new strategies to treat the luminal androgen receptor subtype of triple negative breast cancer with drugs that inhibit the androgen receptor activity. However, their therapeutic value is limited, and effective treatment for luminal androgen receptor subtype of triple negative breast cancer has not yet been achieved. So scientists have worked to identify new targets that can inhibit proliferation, invasion, and metastasis of the luminal androgen receptor subtype of triple negative breast cancer.
Further studies are required to verify whether this regulation of cell cycle progression occurs in other cell types. The team knows that AIbZIP is highly upregulated in many cancers, including luminal and HER2-positive breast cancer. The novel pathway they have discovered may contribute to cancer treatments in other cancer types besides triple negative breast cancer.
Looking ahead, the team sees more work to be done. They want to check the expression level of AIbZIP in tumors derived from triple negative breast cancer patients to confirm the robust link between AIbZIP and the development of triple negative breast cancer. “If we can downregulate the AIbZIP activity, it may lead to develop the novel therapeutic strategy against triple negative breast cancer,” said Imaizumi.
The research team includes Taichi Ito, Atsushi Saito, Yasunao Kamikawa, Nayuta Nakazawa, and Kazunori Imaizumi from the Department of Biochemistry, Graduate School of Biomedical and Health Sciences, Hiroshima University, Japan.
The research is funded by JSPS KAKENHI; Takeda Science Foundation; MSD Life Science Foundation; The UBE Foundation; the Program of the Network-type Joint Usage/Research Center for Radiation Disaster Medical Science, Analysis Center of Life Science, the Natural Science Center for Basic Research and Development, Hiroshima University.
###
About Hiroshima University
Since its foundation in 1949, Hiroshima University has striven to become one of the most prominent and comprehensive universities in Japan for the promotion and development of scholarship and education. Consisting of 12 schools for undergraduate level and 4 graduate schools, ranging from natural sciences to humanities and social sciences, the university has grown into one of the most distinguished comprehensive research universities in Japan. English website: https://www.hiroshima-u.ac.jp/en
Journal
Molecular Cancer Research
DOI
10.1158/1541-7786.MCR-23-0629
Article Title
AIbZIP/CREB3L4 Promotes Cell Proliferation via the SKP2-p27 Axis in Luminal Androgen Receptor Subtype Triple-Negative Breast Cancer
Article Publication Date
18-Jan-2024