Based on non-invasive, easy-to-manipulate near-infrared (NIR) laser irradiation, researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences and the University of the Chinese Academy of Sciences (UCAS) have developed a flexible and potent design for “on-demand” whole tumor cell vaccine (TCV).
Credit: MENG Jiaqi
Based on non-invasive, easy-to-manipulate near-infrared (NIR) laser irradiation, researchers from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences and the University of the Chinese Academy of Sciences (UCAS) have developed a flexible and potent design for “on-demand” whole tumor cell vaccine (TCV).
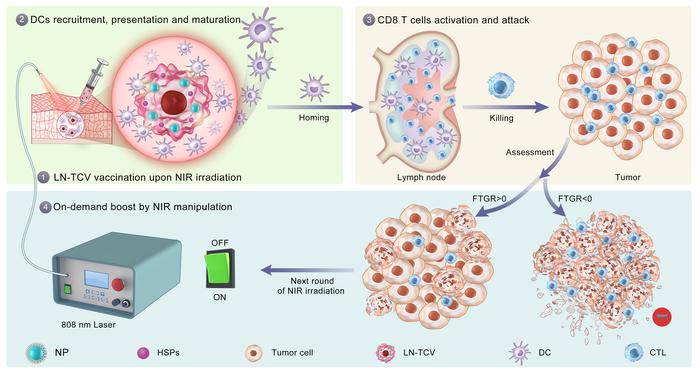
The concept involves loading tumor cells with photothermal nanoparticles, irradiating them with NIR laser irradiation, harvesting and killing the tumor cells to prepare the TCV, then injecting the vaccine into the individual. After a single injection, additional NIR irradiation is applied to the vaccination site. The nanoparticles in the TCV respond to NIR irradiation by becoming hyperthermia, thus boosting the immune response. This on-demand boost to the immune response helps to effectively suppress tumor growth.
The study was published in Nature Communications on July 26.
Tumor vaccines have long been recognized as promising tools for cancer immunotherapy since they utilize the body’s immune system to fight tumor cells. However, among tumor vaccines, TCVs are distinctive because they contain all the antigens in the patient’s own tumor cells not just a select group. As such, TCVs may more easily induce an immune response in the patient since they respond to a broader range of antigens than do ordinary tumor vaccines.
To enhance the immunogenicity of the inactivated tumor cells in vaccines, adjuvant-related methods have been explored. However, most approaches involve complex and time-consuming processes. Another challenge with TCVs is the need for multiple dosing as well as the absence of personalized regimens.
Responding to these deficiencies, researchers have been eager to develop efficient, single-dose TCVs that allow an on-demand boost to the immune response in order to match the heterogeneous immune responses of individual patients.
“In this study, we developed a TCV that supports a single injection-multiple irradiation strategy for on-demand manipulation of the local immune response,” said MENG Jiaqi, first author of the paper.
In preparing the vaccine, the researchers initially loaded photothermal nanoparticles into tumor cells. NIR laser irradiation subsequently induced tumor cells to overexpress heat shock proteins (HSPs) as endogenous adjuvants. The tumor cells were then inactivated through a freeze-thaw process before preparing the vaccine (TCV).
After a single vaccination, the researchers applied NIR laser irradiation at the vaccination site, thus producing locally induced mild inflammation. This promoted the recruitment, activation, and presentation of dendritic cells which then activated T cells in the lymph node for subsequent tumor cell killing.
Prof. TIAN Zhiyuan from UCAS noted that very low-power NIR laser irradiation could generate “enough” local hyperthermia to promote this inflammatory process.
“For monitoring tumor growth rate, we proposed an indicator—the fluctuation of tumor growth rate (FTGR),” said Prof. MA Guanghui from IPE, “FTGR could provide the standard for rational on-demand boost of immune response via repeated NIR laser irradiations at the vaccination site.”
The researchers demonstrated the efficacy of this on-demand TCV strategy using various murine xenograft models derived from tumor cell lines (including triple-negative breast cancer, colon carcinoma, lung carcinoma, and pancreatic carcinoma) and most notably a xenograft—in a murine model with a humanized immune system—that was derived from a pancreatic cancer patient.
“The on-demand TCV strategy has shown good flexibility and potent therapeutic efficacy, and although currently in the preclinical research stage, it holds great potential for future clinical applications,” said Prof. WEI Wei from IPE.
A peer reviewer from Nature Communications said, “The therapeutic data appear promising.” Another reviewer emphasized that “the approach described here is sufficiently innovative and its versatility, based on the possibility to carry out multiple rounds of laser irradiation according to the disease progression, is particularly promising and attractive.”
Journal
Nature Communications
DOI
10.1038/s41467-023-40207-y
Article Title
Generation of whole tumor cell vaccine for on-demand manipulation of immune responses against cancer under near-infrared laser irradiation
Article Publication Date
26-Jul-2023



