In a groundbreaking advance poised to transform liver disease research and regenerative medicine, scientists at Keio University have successfully generated human adult hepatocyte organoids exhibiting mature metabolic functions. These miniature, three-dimensional cultures of liver cells display complex liver activities previously unattainable in laboratory settings, marking a critical leap forward in recapitulating the liver’s intricate biology outside the human body.
One of the long-standing challenges in hepatology and tissue engineering has been the liver’s exceptional complexity. Unlike many organs, the liver performs a vast repertoire of bioactive and metabolic processes, from glucose regulation to bile acid secretion. Replicating this multifaceted functionality in vitro has been hindered by the liver’s demanding energy requirements and the difficulty of sustaining hepatocyte function. Typically, isolated hepatocytes undergo phenotypic changes rapidly when cultured, often transdifferentiating into cholangiocyte-like cells that line bile ducts, resulting in loss of the hepatocyte’s intrinsic metabolic capabilities within one to two weeks.
The innovative research team, led by Ryo Igarashi and Mayumi Oda, overcame this barrier by utilizing cryopreserved adult human hepatocytes sourced directly from patients. Their pivotal discovery centered on the application of oncostatin M, a cytokine involved in inflammatory signaling pathways that had not previously been leveraged in organoid culture systems. Treatment with oncostatin M triggered an extraordinary proliferation phase, achieving a million-fold expansion in organoid numbers. This unprecedented growth contrasts starkly with previous methodologies, which struggled to maintain viable hepatocyte populations beyond short timeframes.
Extended cultivation saw these hepatocyte organoids maintain their viability and proliferative capacity for over three months, surviving up to six months without losing the potential to differentiate. This longevity is critical, as it allows researchers to conduct extended functional studies and disease modeling that were previously impossible due to rapid cell decline. The team also pioneered a chemically-defined hormonal differentiation protocol that stimulates hepatocyte maturation. Post differentiation, the organoids began expressing key liver functions, including the synthesis and secretion of glucose, urea, cholesterol, triglycerides, and bile acids—broadcasting a level of metabolic activity comparable to in vivo human hepatocytes.
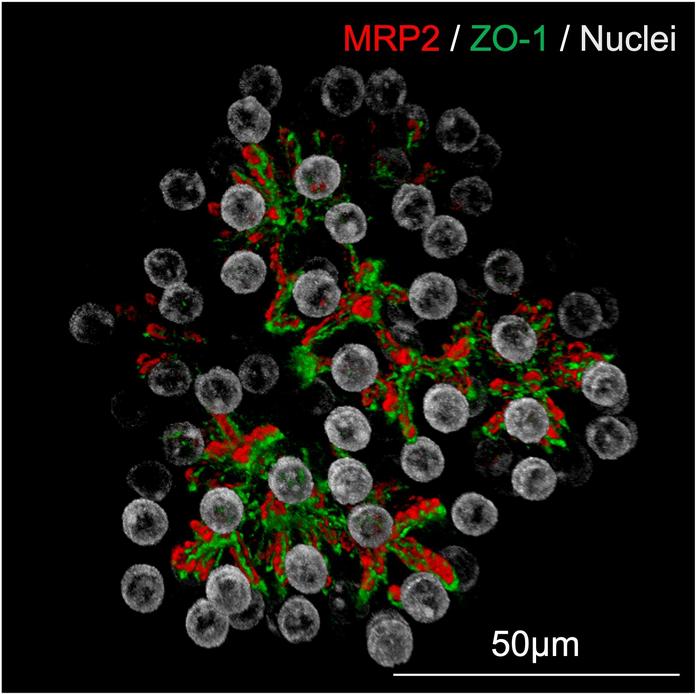
Particularly notable was the organoids’ formation of bile canaliculi-like networks—microscopic tubular structures that enable the transport of bile acids—mimicking the liver’s native architecture. This functionality is essential for modeling hepatobiliary diseases and evaluating drugs that target bile acid metabolism. Levels of albumin secretion, an essential plasma protein responsible for maintaining oncotic pressure and transporting various substances, not only matched but surpassed those reported in existing hepatocyte culture systems.
The identification of oncostatin M as a key modulator of hepatocyte proliferation represents more than just a technical milestone; it uncovers new molecular underpinnings of liver biology. According to senior researcher Toshiro Sato, this discovery expands the compendium of factors capable of ‘unlocking’ the regenerative and differentiation potential of adult liver cells. Previously, only a handful of molecules were known to induce organoid growth, but oncostatin M provides a novel avenue for the creation of diverse, functional liver tissue models.
The translation of this technology into preclinical models further demonstrated its therapeutic promise. When transplanted into immunocompromised mice with impaired liver function, the human hepatocyte organoids engrafted successfully, replacing lost liver cells and restoring fundamental liver functionalities. This achievement addresses the critical bottleneck in liver transplantation—the scarcity and fragility of donor organs. Unlike donated whole organs that must be transplanted rapidly post-harvest, hepatocyte organoids derived from frozen cells can potentially be expanded on demand, circumventing logistical and preservation challenges.
Moreover, this method may revolutionize regenerative therapies by enabling the generation of large quantities of functional liver tissue. Sato emphasizes that scaling up organoid proliferation to match the size and metabolic demand of a human liver remains a formidable hurdle but one with transformative potential. Should this be realized, it could redefine transplantation medicine, offering new lifelines to patients suffering from end-stage liver disease or genetic hepatic disorders.
Beyond transplantation, the hepatocyte organoids hold immense promise for pharmaceutical research. Traditional drug toxicity assessments rely on freshly isolated human hepatocytes, which lose functionality rapidly and vary considerably between batches. This variability complicates the development pipeline and inflates costs due to inconsistent experimental outcomes. In contrast, organoids provide a renewable, consistent source of metabolically active human liver cells, enabling more reliable drug screening, especially for hepatotoxic compounds.
The organoids have also proven superior in disease modeling. For example, they intrinsically synthesized lipids and, upon administration of therapeutic agents targeting metabolic-associated steatotic liver disease (MASLD), these lipid stores diminished accordingly. This contrasts with previous studies that introduced lipids artificially, offering less physiologically relevant models. Moreover, the team successfully employed gene-editing techniques to replicate pathological states such as ornithine transcarbamylase (OTC) deficiency—a rare, inherited disorder disrupting the urea cycle—thereby modeling genetic liver diseases with unprecedented fidelity.
Looking forward, researchers recognize the importance of enhancing organoid complexity. Incorporating additional liver cell types, such as Kupffer cells (resident macrophages), liver sinusoidal endothelial cells, and hepatic stellate cells, is vital to recapitulate the full cellular interplay that underpins liver physiology and immune responses. Furthermore, intensifying proliferative capacity beyond current levels remains a priority to meet the substantial cellular quantities demanded in clinical applications.
In sum, this innovative study represents a significant stride toward mimicking the human liver’s complexity in a laboratory platform. By integrating novel cytokine signaling pathways, advanced differentiation protocols, and precise gene editing, the researchers at Keio University have opened new horizons in personalized medicine, drug discovery, and regenerative therapies. As liver diseases continue to impose a global health burden, such cutting-edge organoid models may soon become indispensable tools in both basic science and translational medicine.
Subject of Research: Cells
Article Title: Generation of human adult hepatocyte organoids with metabolic functions
News Publication Date: April 16, 2025
Web References: http://dx.doi.org/10.1038/s41586-025-08861-y
Image Credits: Toshiro Sato from Keio University
Keywords: hepatocyte organoids, liver regeneration, oncostatin M, organoid proliferation, metabolic functions, liver disease modeling, MASLD, gene editing, urea cycle disorder, transplantation, regenerative medicine
Tags: challenges in tissue engineeringcomplex liver biology replicationcryopreserved human hepatocyteshepatocyte culture advancementshepatocyte functionality preservationinflammatory signaling in organoidsliver disease research innovationsmetabolic function in vitrominiature liver organoidsoncostatin M applicationorganoid technology in hepatologyregenerative medicine breakthroughs