Decorating the outside of cells like tiny antenna, a diverse community of sugar molecules acts like a telecommunications system, sending and receiving information, recognizing and responding to foreign molecules and neighboring cells.
“The sugar part of our biomembranes are as crucial to our health as our DNA, and yet we know almost nothing about it,” said Virgil Percec, a professor of chemistry in the University of Pennsylvania School of Arts and Sciences.
Part of the reason cell membrane sugars, called glycans, are so poorly understood is that scientists were unable to accurately model them until last year, when Percec’s lab devised a way of programming artificial membranes with a precise number and spatial arrangement of sugars.
Now, as a proof-of-concept for their new model, the team has tested its interactions with galectin-8, a cell signaling protein that, when mutated, may contribute to rheumatoid arthritis. Gal-8 is one of a large family of growth-regulatory proteins the team is testing their model against. By modifying a single building block in Gal-8’s structure, exactly as nature does in a portion of the population, the researchers dramatically impaired its ability to communicate with the artificial membrane, suggesting a possible molecular basis for the disease.
Percec’s new study demonstrates how researchers can use this membrane model to examine the interactions of cell surfaces with other biological molecules, with far ranging applications in medicine, biochemistry and biophysics.
“There are lots of membrane sugar-protein interactions that are important for disease,” Percec said. “Now, we have the critical tool we need to develop these disease models.”
Other team members from Penn include postdoctoral chemistry researchers Shaodong Zhang and Ralph-Oliver Moussodia. They collaborated with Temple University’s Michael Klein, as well as Sabine Vértesy and Sabine André and Hans-Joachim Gabius of Ludwig-Maximillians University in Munich.
The study was published in The Proceedings of the National Academy of Sciences.
Cell membranes are composed of two layers of fatty molecules known as phospholipids, each of which has a water-loving head and a water-repellant tail. The simplest form of a membrane, called a liposome or vesicle, will self-assemble when its phospholipid building blocks are placed in water. But vesicles are difficult to produce in the lab and don’t remain stable for long. For decades, these challenges hindered scientific efforts to create artificial membranes for research.
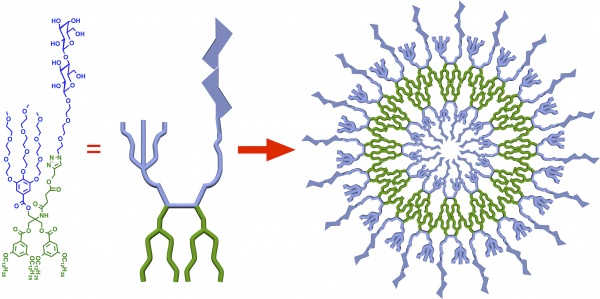
But in 2010 Percec and his lab discovered they could produce stable, self-assembling vesicles by replacing phospholipids with a class of molecules called amphiphilic Janus dendrimers, which have water-loving and water-hating branches, instead of heads and tails. Not only are dendrimer-based vesicles much easier to produce, their size, number of functional ends-groups and the number of concentric layers they contain can be precisely tuned.
“This was a big advance. It provided us the tool we were looking for while saving a huge amount of work,” Percec said. “The next step was to ask ‘can we add surface sugars to it?’”
Early efforts to mimic membrane surfaces in the lab were crude and simplistic, with no control over the number or distribution of sugars. That posed a major limitation to researchers, who need an accurate representation of these surfaces to study how other cells, proteins or viruses, will interact with them.
Building off their dendrimer-based vesicles, Percec’s lab constructed a library of amphiphilic glycodendrimer molecules: dendrimers with chemically bonded glycan sugars. By diluting these glycodendrimers to a series of different concentrations in an organic solvent and injecting them in water, the team found they could program vesicles, called glycodendrimersomes, with different surface sugar topologies. Details of this work were published in 2013 in the Journal of the American Chemical Society.
“As our molecules self-assemble, the vesicles formed have a precise number and spatial arrangement of the sugars, something never possible before,” Percec said.
One of the most important roles membrane sugars play is receiving messages from signaling proteins and communicating those messages to the cell. Many diseases are thought to be the result of communication errors that arise when a signaling protein incurs a mutation or the membrane’s glycan structure is altered. To demonstrate the utility of their new model, the researchers studied how mutant varieties of Gal-8 interacted with a custom artificial membrane containing Gal-8’s specific binding sugars. By modifying a single amino acid in the protein’s structure, as occurs naturally in human populations, they could significantly impair Gal-8’s ability to bind to the membrane.
“By testing this model with a sugar binding protein of human origin, we show that single mutation of an amino acid from a giant protein structure can induce a dramatic change in its interactions with the cell,” Percec said. “This demonstrates just how efficient and sensitive a model this is for biological membranes.”
In the future, the team will continue to develop and refine their glycodendrimersomes models, building membranes of increasing complexity and studying how membrane functions are affected. Besides Gal-8, there are many other biologically interesting signaling proteins, which researchers can now study using a robust and customizable membrane model.
Percec’s glycodendrimersome research is housed at Penn’s interdisciplinary Laboratory for Research on the Structure of Matter and in his own laboratory. Through the LRSM, Percec is collaborating with researchers in bioengineering, computational biology, biology, biophysics and biomedicine who are interested in using his programmable membranes for a variety of purposes, from visualizing the interactions between viruses and cells to developing biological capsules for vaccine and drug delivery.
“A biomembrane with a programmable surface topology is a tool to answer almost any question in cell biology,” Percec said.
Story Source:
The above story is based on materials provided by Penn News