Credit: Graphic: Bernd Fakler
Rapid communication of neurons in the brain, as well as the ability to learn, fundamentally rely on neurotransmitter receptors located in the contact sites of neurons, the synapses. The most important receptors in the mammalian brain are glutamate receptors of the AMPA-type (AMPAR) that generate the electrical signal required for fast communication between neurons. The number of AMPARs is modulated by the degree of a synapse’s activity: As it learns, the number of AMPARs increases, thus making synaptic signal transduction more reliable and driving synaptic plasticity that promotes memory formation. Fundamental requirement for this synaptic plasticity is the efficient assembly of AMPARs from different protein subunits in the endoplasmic reticulum (ER) of nerve cells, for which little or no information has been avaible so far.
For the first time, a team of neurobiologists from Freiburg headed by Prof. Dr. Bernd Fakler from the Institute of Physiology, in cooperation with colleagues from the Goethe University Frankfurt and the Max Planck Institute for Medical Research in Heidelberg, has been able to show that AMPARs are assembled from main and auxiliary subunits in a step-by-step process much like on an assembly line. The individual stages are carried out by different ER-resident proteins and protein complexes. Disturbance of this assembly by mutations in the assembly line elements in humans or by their targeted genetic inactivation – knock-out – in mice, leads to massive impairment of synaptic signal transduction and learning. Conversely, the increase in receptor production through overexpression of the assembly line proteins leads to increased plasticity of the synapses. The scientists recently published these results in the journal Neuron.
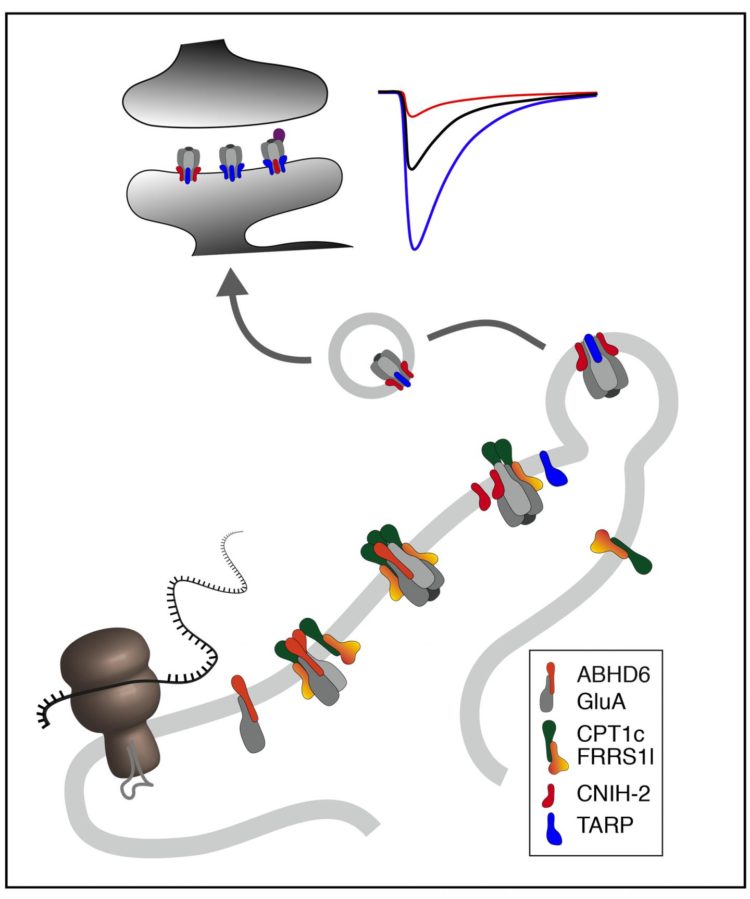
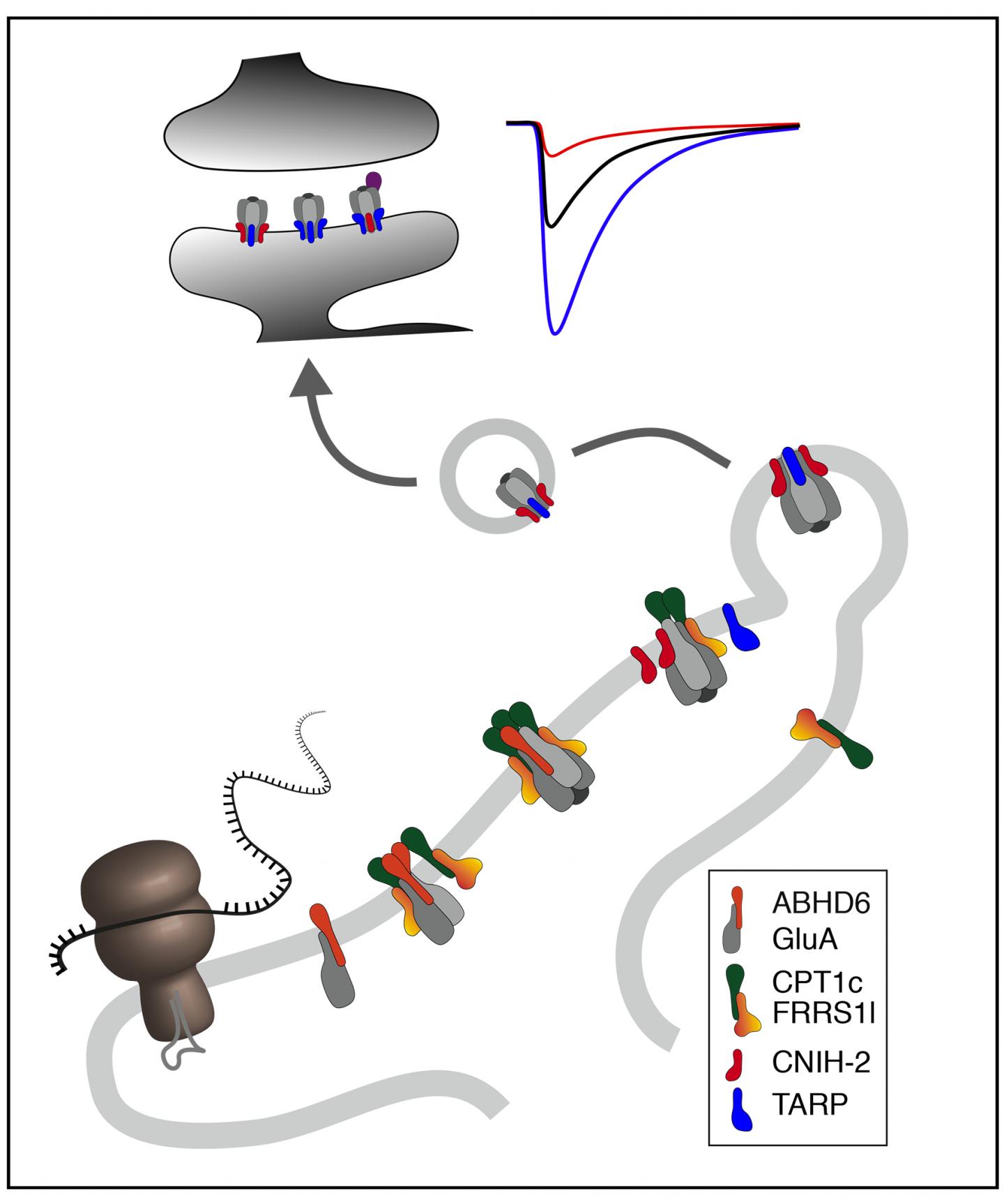
Using high-resolution proteomic techniques, the researchers have identified proteins in the ER membranes of neurons that are required for the assembly of functional AMPARs from four pore-forming subunits and four auxiliary subunits: The first building block, the proteins ABHD6 and PORCN, protects the individual pore-forming GluA subunits from premature degradation. The second building block, a complex of the proteins FRRS1l and CPT1c, assembles four GluA-protein into a receptor channel and prepares their association with the four auxiliary subunits, the cornichon or TARP proteins. This final step dissociates the FRRS1l-CPT1c complex and enables export of the functional AMPARs from the ER and their transport into the synapses.
The individual steps along this assembly line are precisely orchestrated and optimized for the efficient assembly of the receptors. If the operation of the assembly line is disturbed, for example by mutation-related loss of function of the FRRS1l protein, this leads to severe dysfunction of the brain in humans, as described by the researchers in an earlier work in 2017: All patients showed severely restricted intellectual abilities with IQ-values below 40, delayed or missing speech development and an increased tendency for epileptic seizures.
Although the newly identified assembly line is specific for AMPARs, the researchers assume that the process of stepwise assembly is exemplary for other membrane proteins and protein complexes mediating information processing in the brain, propagation of excitation and/or substrate transport in other types of cells.
###
The scientists Drs. Jochen Schwenk, Sami Boudkkazi, Maciej Kocylowski and Bernd Fakler work at the Institute of Physiology. Fakler is a member of the Clusters of Excellence in biological signalling research CIBSS and BIOSS at the University of Freiburg.
Original publivation:
Schwenk, S. Boudkkazi, M. Kocylowski, A. Brechet, G. Zolles, T. Bus, K. Costa, W. Bildl, A. Kollewe, J. Jordan, J. Bank, W. Bildl, R. Sprengel, A. Kulik, J. Roeper, U. Schulte, and B. Fakler (2019): An ER assembly line of AMPA-receptors controls excitatory neurotransmission and its plasticity. In: Neuron. DOI: https:/
Contact:
Institute of Physiology, Department II
Clsuters of Excellence in biological signalling studies CIBSS and BIOSS
University of Freiburg
Media Contact
Bernd Fakler
[email protected]
49-761-203-5175
Related Journal Article
http://dx.