In a groundbreaking study led by researchers at the Johns Hopkins Kimmel Cancer Center and the Johns Hopkins Bloomberg School of Public Health, scientists have uncovered crucial molecular mechanisms underlying a rare and aggressive form of kidney cancer known as translocation renal cell carcinoma (tRCC). This malignancy develops through chromosomal rearrangements that fuse the gene TFE3 with other distinct partner genes, giving rise to novel fusion proteins that significantly alter cellular behavior. The study elucidates how these TFE3 fusion proteins assemble into microscopic liquid condensates within the nucleus, where they orchestrate the activation of oncogenic gene programs, thereby driving cancer progression.
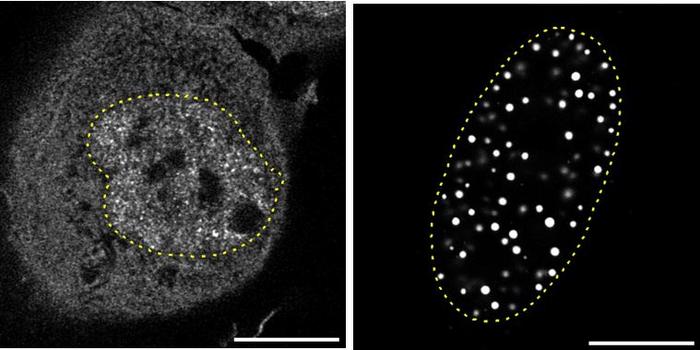
At the heart of this discovery lies the phenomenon of liquid-liquid phase separation, a biophysical process increasingly recognized as fundamental to cellular organization. The researchers demonstrated that unlike the normal TFE3 protein, which diffusely distributes throughout the cell nucleus, the aberrant TFE3 fusion proteins coalesce into dense, droplet-like condensates adjacent to DNA. These condensates function as dynamic hubs that recruit co-regulatory proteins and chromatin remodeling factors, effectively rewiring the epigenetic landscape to favor the transcriptional activation of genes that promote tumor growth and metastasis.
The team specifically focused on two of the most prevalent TFE3 fusion variants involving NONO and SFPQ gene partners, which collectively represent approximately 40% of all TFE3 rearrangements seen in tRCC patients. By tagging these fusion proteins with fluorescent markers and visualizing them in live patient-derived cancer cells, the scientists observed the temporal dynamics of droplet formation and dissolution. Crucially, these condensates were found to sequester both histone modification enzymes and transcriptional activators, indicating a direct mechanistic link between condensate assembly and chromatin accessibility.
Chromatin, the highly organized structure of DNA and proteins within the nucleus, regulates gene expression by modulating the exposure of DNA sequences to the transcriptional machinery. In the chromatin “beads-on-a-string” model, tightly wrapped DNA around nucleosomes corresponds to gene repression, while relaxed or open chromatin permits gene activation. The TFE3 fusion condensates appear to manipulate this structural equilibrium, chemically modifying histone tails to promote the opening of chromatin at specific loci. This epigenomic reprogramming facilitates the upregulation of genes that enhance cellular proliferation and motility, key hallmarks of cancer invasiveness.
Collaborating closely, co-investigator Eneda Toska, Ph.D., an assistant professor of oncology, provided essential insights into the fusion proteins’ interaction with chromatin. Her team utilized advanced assays to map genome-wide changes in chromatin accessibility and found distinct patterns of gain and loss at enhancer and promoter regions targeted by the TFE3 fusions. This targeted rewiring of the chromatin landscape suggests that fusion protein condensates act as master regulators, selectively activating oncogenic pathways while potentially repressing tumor-suppressive genes.
Intriguingly, the structural integrity of these nuclear condensates was shown to depend on a specialized domain within the fusion proteins forming a coiled-coil motif—an alpha-helical structure that mediates protein-protein interactions. Deletion or mutation of this segment disrupted condensate formation, abrogated the fusion proteins’ ability to induce chromatin remodeling, and, importantly, negated the activation of cancer-driving genes. These findings underscore the pivotal role of phase separation-mediated condensate assembly in the oncogenic function of TFE3 fusion proteins and suggest potential therapeutic targets.
The implications of these results extend beyond tRCC, as fusion genes and protein condensates are increasingly implicated in a variety of cancers. Senior author Danfeng “Dani” Cai, Ph.D., posits that other fusion gene-driven malignancies such as Ewing sarcoma and certain leukemias may employ analogous mechanisms involving liquid-liquid phase separation to regulate gene expression. Understanding these biophysical underpinnings opens a promising avenue for developing treatments that specifically disrupt aberrant condensate formation, thereby silencing cancer-promoting gene networks without broadly affecting normal cellular functions.
Currently, there are no standard treatments for translocation renal cell carcinoma, rendering these mechanistic insights particularly critical. Disrupting the formation or stability of TFE3 fusion condensates could represent a novel therapeutic strategy. The research team envisions future drug discovery efforts focused on identifying small molecules capable of interfering with condensate assembly or destabilizing the protein interactions that sustain these oncogenic droplets. Such targeted approaches would offer precision medicine options for patients with this rare but aggressive kidney cancer subtype.
This study not only reveals fundamental aspects of cancer biology but also exemplifies the growing importance of interdisciplinary approaches combining molecular biology, biochemistry, structural biology, and epigenetics. The researchers employed state-of-the-art imaging techniques, genome-wide chromatin profiling, and protein engineering to dissect the complex interplay between gene rearrangements and nuclear organization. Their integrative methodology sets a new standard for investigating the consequences of fusion gene events in cancer.
The discovery that fusion protein-driven condensates act as epigenetic architects advancing tumorigenesis adds to the expanding paradigm in which membraneless organelles govern key regulatory processes within cells. These dynamic condensates enable spatial and temporal control over gene activation, a feature that cancer cells exploit to gain proliferative and invasive advantages. Targeting such condensates offers a disruptive innovation in cancer therapeutics, moving beyond traditional enzyme inhibition to the modulation of higher-order protein assemblies.
As the research community continues to unravel the biophysical and molecular signatures of fusion oncoproteins in tRCC and beyond, this work lays a critical foundation for translating basic science into clinical interventions. The collaboration among Johns Hopkins teams, supported by various grants including from the National Institutes of Health and the Department of Defense, illustrates the power of concerted efforts to tackle rare but formidable cancers through precise mechanistic understanding.
In summary, the identification of liquid droplets formed by TFE3 fusion proteins and their role in reprogramming chromatin accessibility provides an unprecedented glimpse into the molecular drivers of translocation renal cell carcinoma. This insight paves the way for innovative therapeutic paradigms aiming to dismantle oncogenic condensates, offering new hope to patients facing cancers currently lacking effective treatment options. As cancer biology embraces the complexity of nuclear condensates, the convergence of molecular detail and clinical urgency heralds a transformative era in precision oncology.
—
Subject of Research: Molecular mechanisms of TFE3 fusion proteins in translocation renal cell carcinoma and their role in cancer progression through phase-separated nuclear condensates.
Article Title: Fusion Protein Condensates Drive Oncogenic Chromatin Remodeling in Rare Kidney Cancer
News Publication Date: April 22, 2025
Web References: https://www.cell.com/cell-reports/fulltext/S2211-1247(25)00310-9
References: So, Lee, Vokshi et al., 2025 Cell Reports 44, 115539
Image Credits: So, Lee, Vokshi et al., 2025 Cell Reports 44, 115539
Keywords: Kidney cancer, translocation renal cell carcinoma, fusion proteins, TFE3, liquid condensates, chromatin remodeling, cancer epigenetics, phase separation, oncology, gene regulation
Tags: cancer progression mechanismschromosomal rearrangements in cancerepigenetic landscape remodelingfusion proteins in oncologyJohns Hopkins Cancer Center studykidney cancer researchliquid-liquid phase separation in cellsmicroscopic liquid condensatesoncogenic gene activationTFE3 gene fusiontranslocation renal cell carcinomatumor growth and metastasis