Credit: Modified from Krüger et al.,
DNA strand breaks can lead to cell death or to mutations and thus contribute in the long term to cancer development or the ageing process. Fortunately, cells possess molecular tools to repair such DNA strand breaks very efficiently. One of them is the enzyme poly(ADP-ribose) polymerase 1 (PARP1), which detects DNA strand breaks and thereby initiates downstream repair processes.
Scientists at the University of Konstanz (working groups of Professor Aswin Mangerich and Professor Alexander Bürkle, Department of Biology, and working group of Professor Karin Hauser, Department of Chemistry) have now been able to visualize in detail, by means of infrared spectroscopy, the biochemical processes that take place at DNA strand breaks involving PARP1, and could consequently provide important insight into the dynamic changes in the protein structure.
###
More detailed information will be available on the website of the University of Konstanz after the embargo has ended: https:/
Key facts:
- New study by Konstanz researchers from the Departments of Biology and Chemistry provides insights into the molecular processes involved in the detection of DNA strand breaks.
- Real-time observations using infrared spectroscopy permit insights into the detection process with the enzyme poly(ADP-ribose) polymerase 1 (PARP1).
- Important findings on molecular processes of medical relevance, for example with regard to the development of cancer and ageing processes or the mode of action of anti-cancer drugs.
- Original publication: A. Krüger, A. Bürkle, K. Hauser and A. Mangerich, “Real-time monitoring of PARP1-dependent PARylation by ATR-FTIR-spectroscopy”, Nature Communications, 1 May 2020. DOI: 10.1038/s41467-020-15858-w.
- Joint study by the research teams of Professor Aswin Mangerich, Professor Alexander Bürkle (both Department of Biology at the University of Konstanz) and Professor Karin Hauser (Department of Chemistry at the University of Konstanz).
- Further information on the preceding study is available in the online magazine of the University of Konstanz, campus.kn: https:/
/ www. campus. uni-konstanz. de/ en/ science/ uncovering-hidden-protein-structures
Note to editors:
A photo is available for download here: https:/
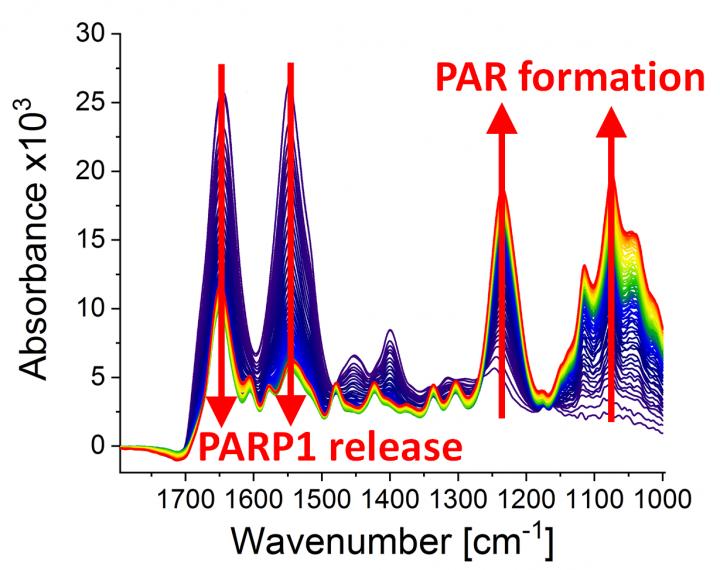
Caption: Infrared spectra at different points of time (0-79 min) after the poly(ADP-ribosyl)ation reaction started due to the addition of PARP1 substrate NAD+. The following can be observed: the dynamic formation of the biopolymer poly(ADP-ribose) (absorption bands at 1236 cm-1 and 1074 cm-1) and the detachment of PARP1 from the DNA strand break (absorption bands at 1645 cm-1 and 1548 cm-1).
Photo: Modified from Krüger et al., “Real-time monitoring of PARP1-dependent PARylation by ATR-FTIR-spectroscopy”, Nature Communications, 1 May 2020. DOI: 10.1038/s41467-020-15858-w.
Contact:
University of Konstanz
Communications and Marketing
Phone: +49 151 27671919
E-Mail: [email protected]
Media Contact
Julia Wandt
[email protected]
Related Journal Article
http://dx.




