Messenger RNA (mRNA) vaccines are revolutionizing the therapy of cancer. It can be flexibly developed in a short period of time, allowing transient expression of multiple antigens for safe and efficient immunization. A diversity of mRNA vaccines is being explored in clinic to benefit patients with cancer. However, the translation of mRNA vaccines is still hampered by multistage delivery barriers before initiating strong immunity, including rapid clearance, poor targeting to lymphoid organs and dendritic cells, catalytic hydrolysis and weak capability to pass through phospholipid bilayers. Besides, vaccination with mRNA alone can barely induce strong immune responses in the absence of adjuvants. It remains challenging to improve cytosolic delivery of mRNA and promote its in vivo vaccination efficacy in combination with adjuvants.
Credit: ©Science China Press
Messenger RNA (mRNA) vaccines are revolutionizing the therapy of cancer. It can be flexibly developed in a short period of time, allowing transient expression of multiple antigens for safe and efficient immunization. A diversity of mRNA vaccines is being explored in clinic to benefit patients with cancer. However, the translation of mRNA vaccines is still hampered by multistage delivery barriers before initiating strong immunity, including rapid clearance, poor targeting to lymphoid organs and dendritic cells, catalytic hydrolysis and weak capability to pass through phospholipid bilayers. Besides, vaccination with mRNA alone can barely induce strong immune responses in the absence of adjuvants. It remains challenging to improve cytosolic delivery of mRNA and promote its in vivo vaccination efficacy in combination with adjuvants.
In past decades, a huge number of nanocarriers have been reported to promote transfection efficacy of nucleic acid drugs or deliver drugs to lymph nodes. These studies provide valuable features including size, surface charge, modification, responsiveness, components and cytotoxicity to achieve the goal for lymph nodes drainage or cytosolic access. Machine learning techniques provide powerful tools for exploring the physicochemical characteristics and biological features of these nanoparticles, and facilitate the design of nanocarriers with high efficiency. Commonly, machine learning models were trained, selected and optimized with high quality and massive datasets from computations and high-throughput experimental data, and in turn guide the rational design, screening and optimization of nanocarriers. By leveraging existing nanocarriers’ databases, machine learning may provide insights into rational design of nanovaccines with high efficiency.
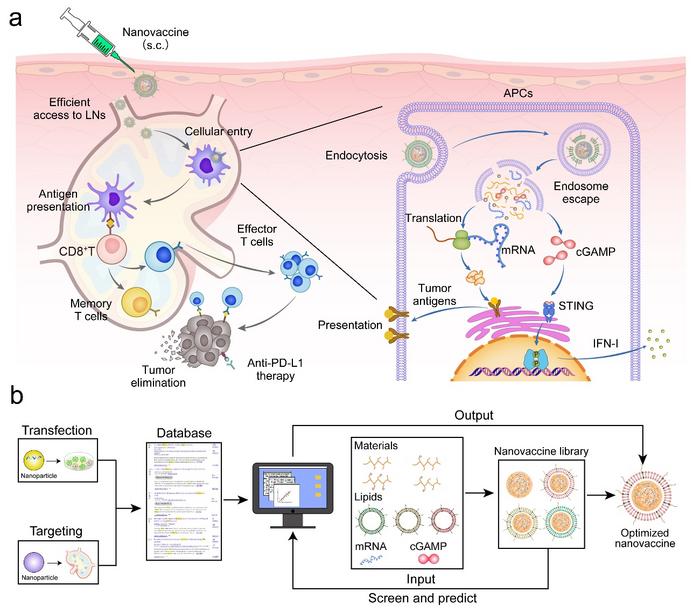
In a new research article published in the Beijing-based National Science Review, scientists from the Chinese Academy of Sciences and Shanghai Jiao Tong University used machine learning to guide the rationalized design of mRNA nanovaccines. This study identified the key parameters of nanovaccines for efficient delivery of mRNA and cGAMP based on a machine learning model from the Nanocarrier Database (2010-2021, web of science). The mRNA/cGAMP nanocomplexes based on phenylboronic acid grafted polyethyleneimine were prepared and further encapsulated with anionic lipids to obtain the nanovaccine. (1) The negative surface charge of the nanovaccine reduces the interaction with negatively charged glycosaminoglycans in matrix and improves accumulation in the lymph nodes. (2) The nanovaccine, after being internalized by the antigen-presenting cells (APCs) in the lymph nodes, promotes the release of mRNA and cGAMP from the endosomes to the cytoplasm, which activates the STING pathway and induces the presentation of tumor antigens. (3) The activation of STING pathway promotes the release of IFN-I, which activates T cell immune response to kill tumor cells and inhibit tumor growth and metastasis. Compared with the mRNA alone, the therapeutic strategy based on this nanovaccine demonstrated stronger anti-tumor effects in melanoma and colorectal cancer models.
###
See the article:
STING agonist-boosted mRNA immunization via intelligent design of nanovaccines for enhancing cancer immunotherapy
https://doi.org/10.1093/nsr/nwad214
Journal
National Science Review
DOI
10.1093/nsr/nwad214