Credit: James Shorter, Perelman School of Medicine, University of Pennsylvania; Cell
PHILADELPHIA – A host of special molecules called nuclear RNA-binding proteins (RBPs), when misplaced outside the nucleus, form the harmful clumps seen in several brain disorders, including frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). "Clumps that form from these disease proteins are composed of sticky fibrils that damage nerve cells," said James Shorter, PhD, an associate professor of Biochemistry and Biophysics in the Perelman School of Medicine at the University of Pennsylvania. "We want to reverse the formation of these clumps and put the RNA-binding proteins back in their proper place, inside the nucleus."
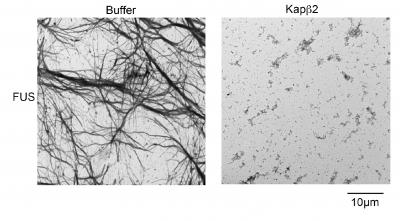
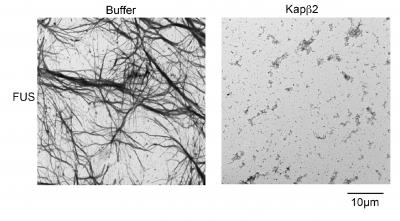
Normally, nuclear-import receptors (NIRs) bind to specific sequences of amino acids on the RBPs, to shepherd them into the nucleus. A team led by Shorter, describes in Cell this week what happened when they added NIRs to aggregates made from TDP-43 and FUS proteins, which are connected to these neurodegenerative diseases.
"When we increased the concentration of NIRs, there were three important and surprising outcomes," said co-first author Lin Guo, PhD, a Target ALS Springboard Fellow. First, clumps of RBPs dissolved in test-tube experiments. Next, NIRs also dissolved cytoplasmic clumps in cells and functional RBPs were returned to the nucleus. And finally, when the expression of NIRs was increased in fruitfly models of disease, lifespan was extended and degeneration was reduced.
"All of this biochemistry was highly unanticipated," said co-first author Henry Wang, an MD/PhD student at Penn. "We didn't suspect that the NIRs would break up the clumps, return the RBPs to the nucleus, and mitigate neurodegeneration."
The team was surprised by how rapidly the NIRs could reverse the formation of the FUS and TDP-43 clumps. The transition from the RBP being dissolved in solution to forming droplet-like structures normally happens in the nucleus as a regular part of RBP function. However, when RBPs, like FUS and TDP-43, are misplaced in the cytoplasm, these phase changes can become aberrant, setting the RBPs on a destructive path, which can be corrected with NIRs. But, NIR expression or activity likely becomes reduced in disease.
'Given this complexity, we are now working to find a way to increase expression or activity of NIRs in neurons with clumps using protein engineering or small-molecule drugs, said coauthor Charlotte Fare, a doctoral student in Shorter's lab.
###
Collaborators include co-senior author J. Paul Taylor, MD, PhD, an investigator with the Howard Hughes Medical Institute and chair of Cell and Molecular Biology at St. Jude Children's Research Hospital; Clotilde Lagier-Tourenne, Mass General Hospital; Yuh Min Chook, University of Texas; and Udai Pandey, University of Pittsburgh.
This research was funded by the National Institute of Health (R21NS090205), Target ALS, the ALS Association, and the The Robert Packard Center for ALS Research at Johns Hopkins.
Penn Medicine is one of the world's leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System, which together form a $7.8 billion enterprise.
The Perelman School of Medicine has been ranked among the top medical schools in the United States for more than 20 years, according to U.S. News & World Report's survey of research-oriented medical schools. The School is consistently among the nation's top recipients of funding from the National Institutes of Health, with $405 million awarded in the 2017 fiscal year.
The University of Pennsylvania Health System's patient care facilities include: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center — which are recognized as one of the nation's top "Honor Roll" hospitals by U.S. News & World Report — Chester County Hospital; Lancaster General Health; Penn Medicine Princeton Health; Penn Wissahickon Hospice; and Pennsylvania Hospital – the nation's first hospital, founded in 1751. Additional affiliated inpatient care facilities and services throughout the Philadelphia region include Good Shepherd Penn Partners, a partnership between Good Shepherd Rehabilitation Network and Penn Medicine, and Princeton House Behavioral Health, a leading provider of highly skilled and compassionate behavioral healthcare.
Penn Medicine is committed to improving lives and health through a variety of community-based programs and activities. In fiscal year 2017, Penn Medicine provided $500 million to benefit our community.
Media Contact
Karen Kreeger
[email protected]
215-459-0544
@PennMedNews
http://www.uphs.upenn.edu/news/