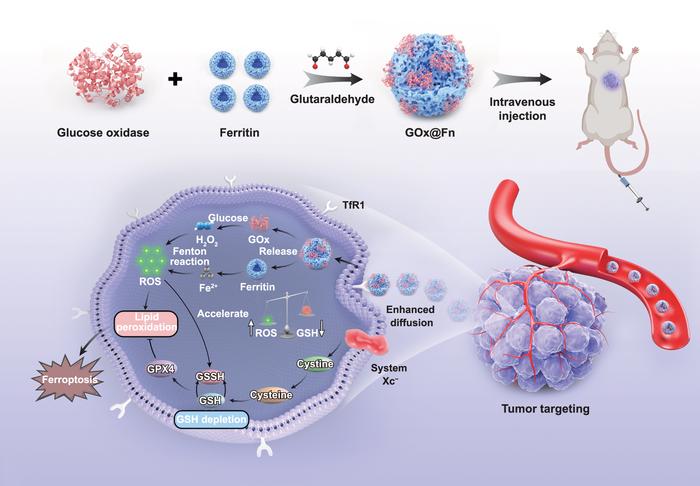
In a groundbreaking development that may revolutionize cancer treatment, a team of researchers at Southern Medical University has engineered a self-propelled ferroptosis nanoinducer capable of penetrating deep into tumor tissues, dramatically enhancing therapeutic efficacy while maintaining biocompatibility. The innovative nanotherapeutic’s ability to navigate the hostile tumor microenvironment and induce ferroptotic cell death represents a significant advancement in the field of targeted cancer therapies, addressing long-standing challenges related to drug delivery and tumor permeability.
Traditional nanoplatforms have long been hampered by their inability to actively penetrate tumor masses, resulting in limited diffusion and consequently poor therapeutic outcomes. This limitation arises primarily from the dense extracellular matrix and high interstitial fluid pressure characteristic of solid tumors, which serve as physical and biochemical barriers to nanoparticle infiltration. Recognizing these obstacles, Professor Yingfeng Tu and his colleagues sought to design a dynamic nanotherapeutic system that could actively propel itself, thereby overcoming the diffusion constraints typical of passive nanomedicines.
.adsslot_Bk3mF0AQW9{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_Bk3mF0AQW9{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_Bk3mF0AQW9{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
Ferroptosis, the form of programmed cell death triggered by iron-dependent lipid peroxidation, has emerged as an effective mechanism for eliminating cancer cells resistant to apoptosis. By integrating ferritin—an iron storage protein—into the nanoparticle, the researchers successfully amplified ferroptotic pathways. Once inside the tumor microenvironment, intracellular uptake of these self-propelled particles initiates ferroptosis, disrupting cellular membranes and impairing essential organelles, including mitochondria and lysosomes. This multi-organelle targeting strategy enhances cytotoxicity and suppresses tumor proliferation more effectively than single-targeted therapies.
Over two years, the team conducted comprehensive assessments of their nanoinducer’s physicochemical properties, motion dynamics, and chemotactic behaviors. Using advanced imaging and tracking techniques, they observed that the nanoinducer actively navigates chemical gradients within tumor tissues, enabling deeper infiltration compared to conventional nanoparticles that rely solely on diffusion. This self-motility translates into homogeneous distribution throughout the tumor mass, maximizing therapeutic payload delivery and minimizing the survival of hypoxic or drug-resistant tumor regions.
In vitro experiments demonstrated pronounced cell death in multiple cancer cell lines upon treatment with the nanoinducer, confirming its potent ferroptosis-inducing activity. Subsequent in vivo studies in murine tumor models revealed significant tumor shrinkage without evident systemic toxicity, highlighting the platform’s translational potential. The authors emphasize that this approach not only boosts antitumor efficacy but also mitigates the side effects commonly associated with chemotherapy and radiotherapy, positioning the nanoinducer as a promising candidate for future clinical applications.
Biocompatibility stands as a defining feature of this technology. The exclusive use of endogenous proteins circumvents immune clearance and adverse reactions, which often complicate nanotherapeutic administration. Given the nanoinducer’s biodegradability, metabolic clearance is efficient, diminishing risks of accumulation and long-term toxicity. This contrasts sharply with many synthetic nanoparticles that persist in the body and can elicit off-target effects, thereby limiting their therapeutic window.
The dual-component system capitalizes on synergistic mechanisms. Glucose oxidase-driven self-propulsion enhances penetration, while ferritin-mediated ferroptosis ensures effective cancer cell eradication. This synergy addresses two critical limitations of current nanotherapeutics: shallow tumor penetration and insufficient induction of programmed cell death pathways. The thoughtful molecular engineering embodied in this platform facilitates active motion and effective biochemical action, a combination rarely achieved in the nanomedicine field.
Looking ahead, the research team is committed to expanding the applicability of their nanoinducer to other malignant cancer types, including notoriously treatment-resistant non-small cell lung cancer. Rigorous preclinical studies and optimization of dosing regimens are underway to support eventual clinical translation. The overarching goal is to develop a versatile, biocompatible nanotherapeutic that can be tailored to diverse oncological contexts, thereby transforming the standard of care.
This pioneering study provides a compelling proof of concept for the integration of self-propulsion and ferroptosis induction in a single, protein-based nanotherapeutic. By surmounting the intrinsic barriers of tumor microenvironments, this technology sets a new benchmark in the targeted delivery of anticancer agents. As the field of nanomedicine advances, such sophisticated platforms are poised to deliver unprecedented precision and efficiency in cancer therapy, with the potential to improve patient survival and quality of life substantially.
The work has been published in the International Journal of Extreme Manufacturing, underscoring the interdisciplinary nature combining nanotechnology, biochemistry, and oncology. The study also highlights the transformative potential of extreme manufacturing techniques in creating multifunctional, dynamic nanostructures tailored for complex biological challenges. The collaboration between engineers, chemists, and clinicians is crucial for driving innovations that cross the boundaries between materials science and medicine.
In sum, the self-propelled ferroptosis nanoinducer from Southern Medical University represents an exciting leap forward in nanotherapeutic design. Grounded in rigorous scientific principles and enabled by novel manufacturing strategies, this platform offers hope for overcoming the long-standing obstacles of tumor penetration and drug resistance. As research continues to unfold, the convergence of active nanomotion and programmed cell death induction heralds a new era in cancer therapeutics.
Subject of Research: Nanotherapeutics; Ferroptosis; Cancer Therapy; Nanoparticle Penetration
Article Title: Self-propelled ferroptosis nanoinducer for enhanced cancer therapy
News Publication Date: 24-Jan-2025
Web References:
https://iopscience.iop.org/journal/2631-7990
http://dx.doi.org/10.1088/2631-7990/ada838
Image Credits: By Wenxin Xu, Hao Tian, Yanzhen Song, Hanfeng Qin, Junbin Gao, Yichi Chen, Weichang Huang, Lin Lin, Haixin Tan, Yicheng Ye, Xiaoting Zhang, Daniela A Wilson, Guang Yang, Fei Peng and Yingfeng Tu
Keywords: Ferroptosis, Nanoparticles, Cancer Therapy, Self-propelled Nanotherapeutics, Tumor Penetration, Biocompatibility, Glucose Oxidase, Ferritin, Programmed Cell Death, Nanomedicine, Tumor Microenvironment, Enzymatic Propulsion
Tags: biocompatible nanoparticle designcancer treatment advancementsengineered nanoinducersferroptosis in cancer therapyglucose oxidase enzymatic activityovercoming drug delivery barriersprotein-powered nanomotorsself-propelled nanotherapeuticssolid tumor penetration challengestargeted cancer therapiestherapeutic efficacy enhancementtumor microenvironment navigation