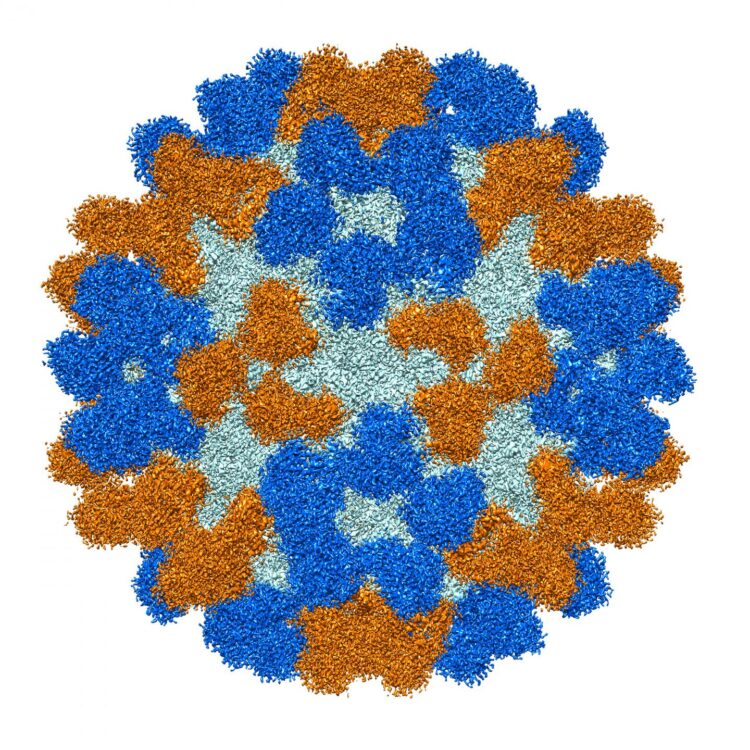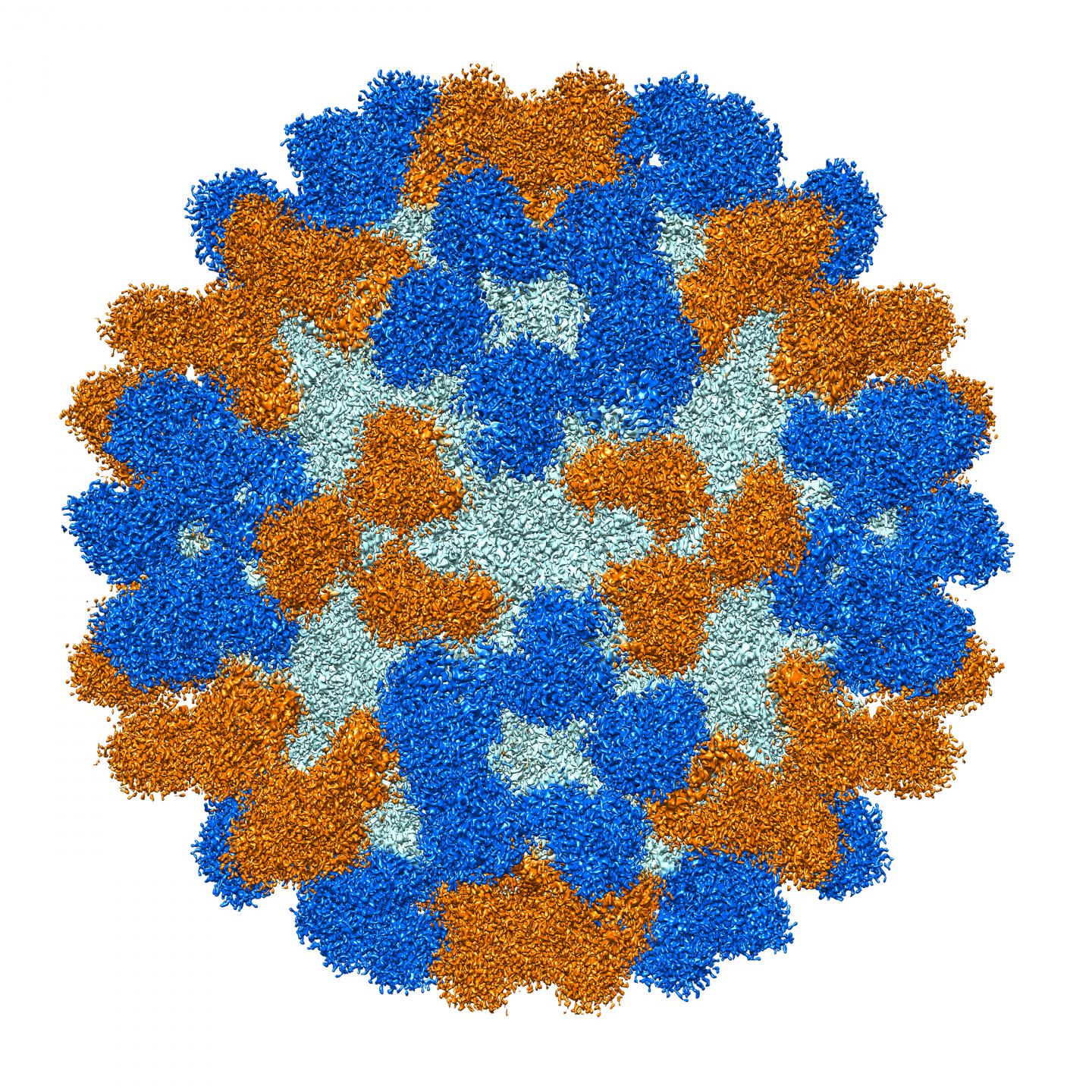
Credit: Purdue University image/Richard Kuhn
WEST LAFAYETTE, Ind. — Researchers at Vanderbilt University Medical Center, Purdue University and the University of Wisconsin-Madison have isolated human monoclonal antibodies that potentially can prevent a rare but devastating polio-like illness in children linked to a respiratory viral infection.
The illness, called acute flaccid myelitis (AFM), causes sudden weakness in the arms and legs following a fever or respiratory illness. More than 600 cases have been identified since the U.S. Centers for Disease Control and Prevention began tracking the disease in 2014.
There is no specific treatment for AFM, which tends to strike in the late summer or early fall and which has been associated with some deaths. However, the disease has recently been linked to a group of respiratory viruses called enterovirus D68 (EV-D68).
Researchers at the Vanderbilt Vaccine Center isolated antibody-producing blood cells from the blood of children who had previously been infected by EV-D68. By fusing the blood cells to fast-growing myeloma cells, the researchers were able to generate a panel of monoclonal antibodies that potently neutralized the virus in laboratory studies.
Colleagues at Purdue determined the structure of the antibodies, which shed light on how they specifically recognize and bind to EV-D68. One of the antibodies protected mice from respiratory and neurologic disease when given either before or after infection by the enterovirus.
Comments from researchers
“We were excited to isolate potent human antibodies that inhibit this devastating polio-like virus, and these studies will form the basis for taking them forward to clinical trials,” Dr. James Crowe, director, Vanderbilt Vaccine Center; Ann Scott Carell Chair and professor of Pediatrics and Pathology, Microbiology and Immunology in the Vanderbilt University School of Medicine.
“Studying infectious disease from a very basic level and applying the results in an animal model of disease is very powerful; hopefully, our studies will translate to a future therapeutic for this disease in children,” Richard Kuhn, Purdue’s Trent and Judith Anderson Distinguished Professor in Science; Krenicki Family Director, Purdue Institute of Inflammation, Immunology and Infectious Disease
###
The study was supported by National Institutes of Health grants HL069765, AI117905, HL070831, AI104317 and AI011219, and the Center for Structural Genomics of Infectious Diseases.
ABSTRACT
Human antibodies neutralize enterovirus D68 and protect against infection and paralytic disease
Matthew R. Vogt1, Jianing Fu2, Nurgun Kose3, Lauren E. Williamson4, Robin Bombardi3,
Ian Setliff5, Ivelin S. Georgiev3,4,5, Thomas Klose2, Michael G. Rossmann2 (deceased), Yury A. Bochkov6, James E. Gern6,7, Richard J. Kuhn2, James E. Crowe Jr.1,3,4,5
1Department of Pediatrics (Infectious Diseases), Vanderbilt University Medical Center, Nashville, Tenn.
2Department of Biological Sciences and Purdue Institute of Inflammation, Immunology, and Infectious Disease, Purdue University, West Lafayette, Ind.
3Vanderbilt Vaccine Center, Vanderbilt University Medical Center, Nashville, Tenn.
4Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, Nashville, Tenn.
5Program in Chemical and Physical Biology, Vanderbilt University, Nashville, Tenn.
6Department of Pediatrics, University of Wisconsin-Madison, Madison, Wisc.
7Department of Medicine, University of Wisconsin-Madison, Madison, Wisc.
Enterovirus D68 (EV-D68) causes outbreaks of respiratory illness, and there is increasing evidence that it causes outbreaks of acute flaccid myelitis (AFM). There are no licensed therapies to prevent or treat EV-D68 infection or AFM disease. We isolated a panel of EV-D68-reactive human monoclonal antibodies that recognize diverse antigenic variants from participants with prior infection. One potently neutralizing cross-reactive antibody, EV68-228, protected mice from respiratory and neurologic disease when given either before or after infection. Cryo-electron microscopy studies revealed that EV68-228 and another potently neutralizing antibody (EV68-159) bound around the fivefold or threefold axes of symmetry on virion particles, respectively. The structures suggest diverse mechanisms of action by these antibodies. The high potency and effectiveness observed in vivo suggest that antibodies are a mechanistic correlate of protection against AFM disease and are candidates for clinical use in humans with EV-D68 infection.
Media Contact
Steve Tally
[email protected]