Degenerative arthritis is no longer exclusive to the elderly population. According to the National Health Insurance Service report covering the years from 2012 to 2022, there has been a 22.8% increase in the prevalence of degenerative arthritis among people in their 20s and 30s. This rise is attributed to prolonged periods of desk sitting and the excessive lifting of heavy sports equipment, both of which can lead to significant cartilage damage. While artificial joints are a common treatment, bacterial infections have posed challenges. However, a recent study has proposed an intriguing solution involving the use of mussels.
Credit: POSTECH
Degenerative arthritis is no longer exclusive to the elderly population. According to the National Health Insurance Service report covering the years from 2012 to 2022, there has been a 22.8% increase in the prevalence of degenerative arthritis among people in their 20s and 30s. This rise is attributed to prolonged periods of desk sitting and the excessive lifting of heavy sports equipment, both of which can lead to significant cartilage damage. While artificial joints are a common treatment, bacterial infections have posed challenges. However, a recent study has proposed an intriguing solution involving the use of mussels.
A collaborative research team, comprising of Professor Hyung Joon Cha from the Department of Chemical Engineering and the School of Convergence Science and Technology and Dr. Hyun Sun Choi from the Department of Chemical Engineering at Pohang University of Science and Technology (POSTECH), and Professor Yun Kee Jo from the Department of Biomedical Convergence Science and Technology of the College of Advanced Technology Convergence at the Kyungpook National University, has successfully developed a coating material for implants. This material, based on mussel adhesion proteins, is designed to release antibiotics in response to bacterial invasion. The research has been recently published in the online edition of Biomaterials, a prominent international journal in the field of biomaterials.
In implant procedures, bacterial infections not only compromise the stability of the implant but also give rise to various complications. Moreover, highly antibiotic-resistant bacteria often lead to recurrent infections even after antibacterial treatment, requiring additional procedures. While there has been active exploration of implant coating materials with antibiotics, numerous challenges have emerged including physical damage to the material during the procedure and potential leakage of antibiotics inside.
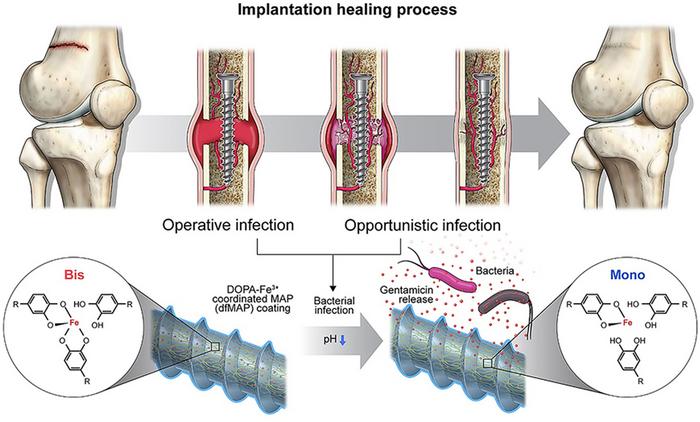
In this research, the team directed their attention to DOPA, one of the amino acids found in mussel adhesion proteins. DOPA, crucial for the robust adhesion observed in mussels, forms potent bonds with metal ions. Its interaction with ferrous metal ions is notable because it weakens as the acidity (pH) decreases. Recognizing that bacterial invasion alters the body’s acidity, the team developed a novel implant coating material.
This material contains antibiotics under normal conditions, but in the event of a bacterial infection and subsequent acidification, it releases 70 percent of the antibiotics within eight hours, effectively eliminating the bacteria. Notably, the material exhibits remarkable durability, showcasing immediate antibacterial efficacy even during the bone regeneration phase (approximately four weeks) following the implant procedure.
The quantity of antibiotics discharged by the material corresponds to the extent of bacterial infection, and the researchers additionally validated the antibacterial efficacy of the coating material based on varying bacterial concentrations. Particularly, the bond between DOPA and iron ions showed remarkable resilience to external physical stimuli, rendering it resistant to abrasion and mechanical loads encountered during the implantation process.
Professor Hyung Joon Cha of the POSTECH who led the study expressed his expectation by saying, “The immediate and sustained antimicrobial effect of the adhesive implant coating material has the potential to significantly enhance the success rate of implant procedures.” Professor Yun Kee Jo of the Kyungpook National University added, explaining the significance of the research, “By releasing antibiotics selectively in response to actual need, this could represent a groundbreaking technology in preventing the emergence of superbacteria in the future.”
The research was conducted with support from the Korea Health Technology R&D Project and the Dentistry Technology R&D Project of the Ministry of Health and Welfare, the Mid-Career Research Program and the Young Researcher Program of the Ministry of Science and ICT, and POSCO Holdings.
Journal
Biomaterials
DOI
10.1016/j.biomaterials.2023.122457
Article Title
Self-controllable proteinic antibacterial coating with bacteria-triggered antibiotic release for prevention of periprosthetic infection
Article Publication Date
2-Jan-2024




