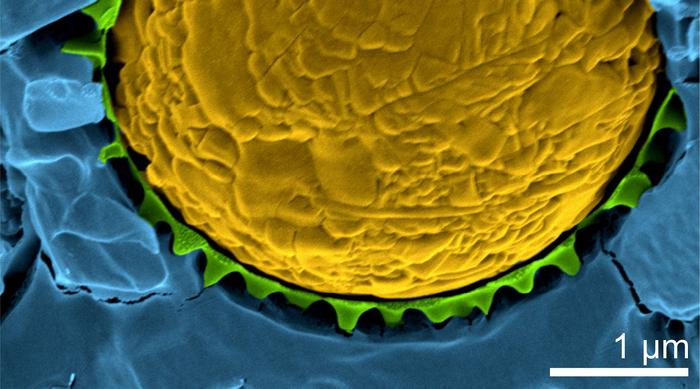
Microgels form a thin protective shell around a droplet until the temperature rises above 32 degrees. Then the microgels shrink and the droplet dissolves in the surrounding liquid. A study by researchers from the University of Gothenburg now reveals the underlying mechanism behind this process. The discovery could revolutionise methods of targeting medicines to specific locations within the body.
Credit: Marcel Rey
Microgels form a thin protective shell around a droplet until the temperature rises above 32 degrees. Then the microgels shrink and the droplet dissolves in the surrounding liquid. A study by researchers from the University of Gothenburg now reveals the underlying mechanism behind this process. The discovery could revolutionise methods of targeting medicines to specific locations within the body.
Emulsions consist of numerous droplets that are present in a liquid without dissolving and mixing with the liquid. For example, milk consists of fat droplets stabilised by milk proteins that are dispersed in water. In many applications such as medicine delivery, it is important to not only maintain the droplet structure but also to be able to control when the droplets dissolve. This is because the encapsulated active ingredients in the droplet should only be released once the medicine has entered the body.
Temperature-sensitive emulsions
Researchers from several universities, including the University of Gothenburg, have introduced a concept of responsive emulsions to control when the droplets dissolve.
“The idea is to stabilise emulsions using temperature-sensitive microgel particles that adapt their shape to the ambient temperature. At room temperature, they swell in water, but above 32°C, they shrink and contract,” explains Marcel Rey, a researcher in Physics at the University of Gothenburg and lead author of the study published in Nature Communications.
Understanding the mechanism
What happens when the temperature rises above 32°C is that the droplets dissolve in the surrounding liquid as they are no longer sufficiently stabilized by the protective microgel shell. While this phenomenon has been known in science for an extended period, the researchers have now uncovered that the fundamental mechanism driving stimuli-responsive emulsions involves morphological changes in the stabilizing microgels.
“The morphological changes in the stabilizing microgels, triggered by external stimuli, play a crucial role in influencing the stability of the associated emulsions. This understanding is fundamental to the design of microgels capable of stabilizing emulsions at room temperature while facilitating dissolution at body temperature,“ explains Marcel Rey.
The stabilising microgels can be regarded as both particles and polymers. The particle character leads to a high stability of the emulsion, while the polymer character makes the microgels responsive to external influences leading to dissolution of the droplets. Achieving temperature-sensitive emulsions necessitates a delicate balance, requiring a minimal particle character for stability and a substantial polymer character for rapid and reliable dissolution of the droplets.
Emulsions can be tailored
“Now that we understand how responsive emulsions function, we can customize them to specific requirements. While our current efforts have been confined to laboratory experiments with temperature dependence, we are actively exploring the development of microgel-stabilized emulsions that respond to the pH of the surrounding fluid,” explains Marcel Rey.
Pharmaceutical research focussing on targeted medicines is crucial. The goal is to deliver medication in a higher concentration to specific diseased areas of the body rather than affecting the entire body.
“Responsive emulsions hold great potential as a precise tool for delivering medicine to specific areas in the body. Although additional research is needed, the future looks promising, and advancements can be expected over the next 10 years,” expresses Marcel Rey.
Scientific article in Nature Communications: Interactions between interfaces dictate stimuli-responsive emulsion behaviour.
Microgels form a thin protective shell around a droplet until the temperature rises above 32 degrees. Then the microgels shrink and the droplet dissolves in the surrounding liquid. A study by researchers from the University of Gothenburg now reveals the underlying mechanism behind this process. The discovery could revolutionise methods of targeting medicines to specific locations within the body.
Emulsions consist of numerous droplets that are present in a liquid without dissolving and mixing with the liquid. For example, milk consists of fat droplets stabilised by milk proteins that are dispersed in water. In many applications such as medicine delivery, it is important to not only maintain the droplet structure but also to be able to control when the droplets dissolve. This is because the encapsulated active ingredients in the droplet should only be released once the medicine has entered the body.
Temperature-sensitive emulsions
Researchers from several universities, including the University of Gothenburg, have introduced a concept of responsive emulsions to control when the droplets dissolve.
“The idea is to stabilise emulsions using temperature-sensitive microgel particles that adapt their shape to the ambient temperature. At room temperature, they swell in water, but above 32°C, they shrink and contract,” explains Marcel Rey, a researcher in Physics at the University of Gothenburg and lead author of the study published in Nature Communications.
Understanding the mechanism
What happens when the temperature rises above 32°C is that the droplets dissolve in the surrounding liquid as they are no longer sufficiently stabilized by the protective microgel shell. While this phenomenon has been known in science for an extended period, the researchers have now uncovered that the fundamental mechanism driving stimuli-responsive emulsions involves morphological changes in the stabilizing microgels.
“The morphological changes in the stabilizing microgels, triggered by external stimuli, play a crucial role in influencing the stability of the associated emulsions. This understanding is fundamental to the design of microgels capable of stabilizing emulsions at room temperature while facilitating dissolution at body temperature,“ explains Marcel Rey.
The stabilising microgels can be regarded as both particles and polymers. The particle character leads to a high stability of the emulsion, while the polymer character makes the microgels responsive to external influences leading to dissolution of the droplets. Achieving temperature-sensitive emulsions necessitates a delicate balance, requiring a minimal particle character for stability and a substantial polymer character for rapid and reliable dissolution of the droplets.
Emulsions can be tailored
“Now that we understand how responsive emulsions function, we can customize them to specific requirements. While our current efforts have been confined to laboratory experiments with temperature dependence, we are actively exploring the development of microgel-stabilized emulsions that respond to the pH of the surrounding fluid,” explains Marcel Rey.
Pharmaceutical research focussing on targeted medicines is crucial. The goal is to deliver medication in a higher concentration to specific diseased areas of the body rather than affecting the entire body.
“Responsive emulsions hold great potential as a precise tool for delivering medicine to specific areas in the body. Although additional research is needed, the future looks promising, and advancements can be expected over the next 10 years,” expresses Marcel Rey.
Scientific article in Nature Communications: Interactions between interfaces dictate stimuli-responsive emulsion behaviour.
Journal
Nature Communications
DOI
10.1038/s41467-023-42379-z
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Interactions between interfaces dictate stimuli-responsive emulsion behaviour
Article Publication Date
23-Oct-2023




