A groundbreaking advancement in the treatment of neuroendocrine tumors has emerged from a recent Phase I clinical trial, heralding a promising new era in targeted cancer therapy. Researchers have reported that combining a radiopharmaceutical agent known as ^177Lu-DOTATATE with the DNA repair inhibitor olaparib is not only feasible but also tolerable for patients afflicted with somatostatin-positive neuroendocrine tumors. This combination, designed to enhance tumor cell eradication, holds significant promise for extending disease control in a patient population faced with limited long-term options.
^177Lu-DOTATATE, or lutetium-177 DOTATATE, embodies a peptide receptor radionuclide therapy (PRRT) that targets neuroendocrine tumor cells by binding to somatostatin receptors, delivering localized radiation directly to malignant tissue. Although ^177Lu-DOTATATE has revolutionized treatment paradigms by inducing durable clinical responses sometimes lasting years, eventual disease progression remains an unabated challenge. Hence, methods to boost its therapeutic efficacy without escalating toxicity are of critical importance.
Addressing this need, the implementation of PARP inhibitors, such as olaparib, offers a compelling biological rationale. PARP enzymes play an essential role in repairing DNA single-strand breaks. Inhibition of these enzymes compromises DNA repair pathways, particularly in cancer cells subjected to DNA-damaging agents like radiopharmaceuticals. The synergistic potential lies in preventing tumor cells from mending radiation-induced DNA damage, thereby amplifying cell death and, subsequently, therapeutic effectiveness.
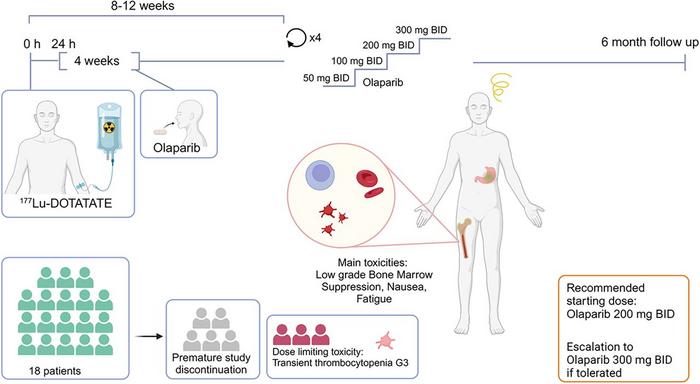
This rationale was rigorously investigated in the LuPARP Phase I clinical trial, spearheaded by Dr. Andreas Hallqvist and colleagues at the Sahlgrenska University Hospital in Gothenburg, Sweden. The study enrolled eighteen patients with somatostatin receptor-positive neuroendocrine tumors who received cycles of ^177Lu-DOTATATE followed by escalating oral doses of olaparib ranging from 50 mg to 300 mg administered twice daily. The primary objective was to assess safety, tolerability, and establish a recommended starting dose for future trials.
The toxicity profile observed during the study was encouraging. The most significant adverse event linked to the combination therapy was thrombocytopenia, a condition characterized by decreased platelet counts, which emerged as the dose-limiting toxicity in three patients at the highest olaparib dose level of 300 mg. Nonetheless, other side effects were predominantly low-grade and manageable, including bone marrow suppression, nausea, and fatigue. This safety data suggests that the combination treatment can be administered with an acceptable risk-benefit ratio.
Importantly, efficacy signals, while preliminary given the Phase I design, were observed. Six months following treatment, a disease control rate of 69% was recorded, indicating that a substantial proportion of patients achieved disease stabilization or response. This early indication highlights the potential of combining targeted radiotherapy with DNA repair inhibition to overcome resistance mechanisms that limit the success of ^177Lu-DOTATATE alone.
From a mechanistic perspective, the ability of olaparib to impede poly(ADP-ribose) polymerase (PARP) enzymes inhibits the repair of single-strand breaks induced by radiation. In neuroendocrine tumor cells treated with ^177Lu-DOTATATE, the persistence of unrepaired DNA lesions leads to double-strand breaks during DNA replication, triggering apoptosis. This biochemical interplay forms the foundation of the observed clinical benefit.
The LuPARP Phase I trial thus stands as a pioneering endeavor marrying nuclear medicine and precision oncology. By leveraging molecular imaging to confirm somatostatin receptor positivity and applying a biologically rational combination, researchers have crafted an innovative treatment modality tailored to the underlying tumor biology. Such approaches epitomize the shift toward personalized medicine, emphasizing therapy customization based on molecular tumor characteristics.
Dr. Hallqvist emphasized that these findings open pathways to smarter cancer treatments that integrate targeted radiotherapy with adjunctive agents designed to enhance efficacy while monitoring and managing adverse effects. This strategy potentially mitigates the limitations of monotherapies and fosters more durable disease control.
The study’s implications extend beyond neuroendocrine tumors, suggesting that similar combinatorial strategies could be employed in other malignancies where targeted radiopharmaceuticals are utilized. The integration of PARP inhibitors could represent a robust platform for amplifying radiotherapy effects, potentially reshaping treatment paradigms across oncology subfields.
Nevertheless, the authors underscore that additional clinical trials, particularly Phase II and III studies, are essential to validate efficacy findings, refine dosing regimens, and fully characterize safety profiles. These future investigations will be critical for translating the LuPARP trial’s promising results into clinical practice, ultimately improving patient outcomes.
In conclusion, the feasibility demonstrated by the combination of ^177Lu-DOTATATE and olaparib marks a significant milestone in neuroendocrine tumor therapy. By strategically disabling tumor DNA repair pathways in concert with receptor-targeted radiotherapy, researchers have illuminated a path toward enhanced, tailored cancer treatments with the potential to extend survival and quality of life for patients.
Subject of Research: Combination therapy using ^177Lu-DOTATATE and PARP inhibitor olaparib in neuroendocrine tumors
Article Title: 177Lu-DOTATATE in Combination with PARP Inhibitor Olaparib Is Feasible in Patients with Somatostatin-Positive Tumors: Results from the LuPARP Phase I Trial
News Publication Date: 1-May-2025
Web References:
https://doi.org/10.2967/jnumed.124.268902
https://jnm.snmjournals.org/
Image Credits: Image created by Elva Brynjarsdóttir, Department of Oncology, Sahlgrenska University Hospital, Gothenburg, Sweden.
Keywords: Medical treatments, Personalized medicine
Tags: ^177Lu-DOTATATE and olaparibclinical trial for neuroendocrine tumorscombination therapy for cancerDNA repair inhibition in cancerenhancing radiopharmaceutical efficacylong-term options for neuroendocrine tumorsneuroendocrine tumor treatmentPARP inhibitors in oncologypeptide receptor radionuclide therapysafe cancer therapiessomatostatin-positive tumor treatmenttargeted cancer therapy advancements