A new investigation led by Tomàs Marquès-Bonet, an ICREA researcher at the IBE (CSIC-UPF) and a professor of Genetics at the Department of Medicine and Life Sciences (MELIS) at Pompeu Fabra University (UPF), Kyle Farh (Illumina), and Jeffrey Rogers (Baylor College of Medicine), combines the genome sequencing of over 800 individuals from 233 primate species, covering nearly half of all existing primate species on Earth, through the study of fossil remains, multiplying by four the number of primate genomes available to date. The study provides new information about primates’ genetic diversity and phylogeny, which is important for understanding and conserving the biodiversity of the closest species to our own.
Credit: Credit: Lukas Kuderna
A new investigation led by Tomàs Marquès-Bonet, an ICREA researcher at the IBE (CSIC-UPF) and a professor of Genetics at the Department of Medicine and Life Sciences (MELIS) at Pompeu Fabra University (UPF), Kyle Farh (Illumina), and Jeffrey Rogers (Baylor College of Medicine), combines the genome sequencing of over 800 individuals from 233 primate species, covering nearly half of all existing primate species on Earth, through the study of fossil remains, multiplying by four the number of primate genomes available to date. The study provides new information about primates’ genetic diversity and phylogeny, which is important for understanding and conserving the biodiversity of the closest species to our own.
By comparing the genomes of 809 nonhuman primate individuals from 233 species to the human genome, the research has identified 4.3 million common missense mutations that affect the composition of amino acids and can alter the function of proteins, leading to many human diseases.
Rare missense mutations can raise the risk of disease
One of the limitations of human and clinical genetics is the current inability to detect, among hundreds of thousands of mutations, the ones that cause diseases. Currently, the genetic causes of many common diseases, such as diabetes and heart disease, are unknown, due either to the lack of genetic information or to the large number of genetic factors involved. Some diseases are thought to originate when a set of genetic variations or mutations with a “mild” effect act together to cause a disease of polygenic origin, like diabetes or cancer.
“6% of the 4.3 million missense mutations identified are abundant in primates and are therefore considered “potentially benign” in human disease, given that their presence is tolerated in these animals”, states Kyle Farh, vice president of Artificial Intelligence at Illumina.
The identification of disease-causing mutations has been achieved thanks to the PrimateAI-3D deep learning algorithm. PrimateAI-3D is an artificial intelligence (AI) algorithm developed by Illumina, the world’s leading company in DNA sequencing, and is a kind of ChatGPT for genetics that uses genome sequences instead of human language.
New insights into primate evolution and human uniqueness
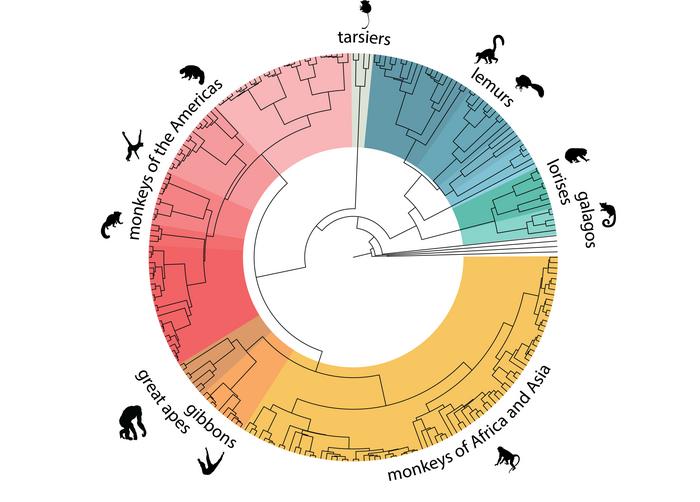
The publication of this project includes the most complete catalogue of primate genomic information produced so far, covering nearly half of all existing primate species on Earth. It contains information of primates from Asia, America, Africa, and Madagascar.
According to Tomàs Marquès-Bonet, “Humans are primates. The study of hundreds of nonhuman primate genomes, given their phylogenetic position, is very valuable for human evolutionary studies, to better understand the human genome and the bases of our singularity, including the bases of human diseases, and for their future conservation”.
These studies have also indicated that the genetics of primates does not always match their taxonomy. We found several cases in which relationships among primate species are best described as complex and network-like rather than simple branching trees.
Another study delves deeper into the evolution of baboon species, a large and diverse group of monkeys, showing that there have been several episodes of hybridization and gene flow among species that were not previously recognized. In addition, we found that yellow baboons from western Tanzania are the first nonhuman primate to have received genetic input from three different lineages. “These results suggest that the population genetic structure and history of introgression among baboon lineages is more complex than was previously thought, and that shows that the baboons are a good model for the evolution of humans, Neanderthals and Denisovans”, says Jeffrey Rogers, an associate professor at the Human Genome Sequencing Center and Dept. of Molecular and Human Genetics at Baylor College of Medicine, who co-led this study.
“Our studies provide clues as to which species are in most dire need of conservation efforts, and could help to identify the most effective strategies to preserve them.”, Lukas Kuderna, first author of one of the studies, asserts.
Lastly, the new genomic catalogue has halved the number of genomic innovations that were believed to be exclusively human. This observation facilitates the identification of mutations that are not shared with primates that may consequently be unique to human evolution and the characteristics that make us human.
Journal
Science
DOI
10.1126/science.abn7829
Method of Research
Experimental study
Subject of Research
Animal tissue samples
Article Title
A global catalog of whole-genome diversity from 233 primate species
Article Publication Date
1-Jun-2023
COI Statement
LFKK, HG, JGS, and KF are employees of Illumina Inc. as of the submission of this manuscript.




