Credit: U.S. Department of Energy, Ames Laboratory
Scientists at the Institute for Cooperative Upcycling of Plastics (iCOUP), an Energy Frontier Research Center led by Ames Laboratory, have discovered a chemical process that provides biodegradable, valuable chemicals, which are used as surfactants and detergents in a range of applications, from discarded plastics. The process has the potential to create more sustainable and economically favorable lifecycles for plastics.
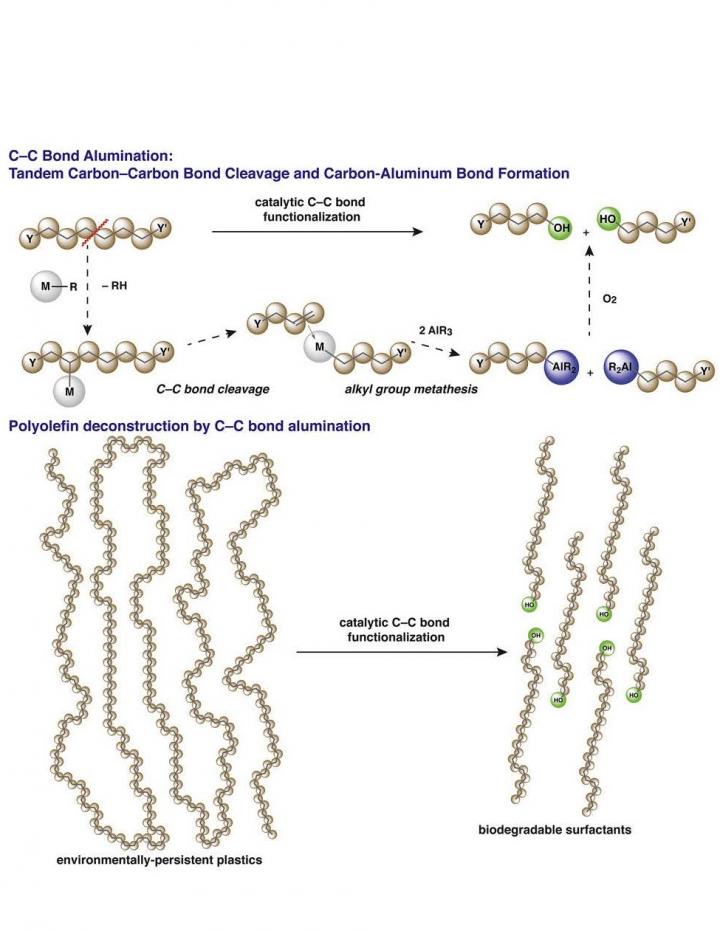
The researchers targeted their work on the deconstruction of polyolefins, which represents more than half of all discarded plastics, and includes nearly every kind of product imaginable– toys, food packaging, pipe systems, water bottles, fabrics, shoes, cars, and furniture.
“Plastics, and especially polyolefins, are materials you could call too successful,” said iCOUP Director Aaron Sadow. “They are fantastic– strong, lightweight, thermally stable, chemically resistant– for all the applications that we use them for, but the problem comes when we don’t need them anymore.”
It’s all in the chemical construction of polyolefin plastics that makes them so tough and durable– long strong chains of carbon-carbon bonds– that also makes them hard to break down. Polyolefins also generally lack the chemical groups which could be targeted in deconstruction processes. Many existing processes to recycle plastic result in less-valuable, less usable components, making the economic feasibility of recycling far less appealing.
The new process uses what science already knows about key steps of polymerization– the assembling of long polymer strands– but in reverse, by breaking some of the carbon-carbon bonds in the chains. Once a few carbon-carbon bonds are broken, the shortened polymer chains transfer to an aluminum end group to form reactive species. The catalysts and reactions for this new process are related to those used in alkene polymerization, leveraging well-understood catalytic chemistry. Finally, the intermediates of this new transformation are easily converted into fatty alcohols or fatty acids, or used in other synthetic chemistry, to create chemicals or materials that are valuable in a whole host of ways: as detergents, emulsifiers, pharmaceuticals, and cosmetics. Because the process is catalytically controlled, desirable product chain lengths can be targeted for synthesis.
The best part about the process is that its end products are biodegradable, unlike polyethylene and polypropylene starting materials.
“Fatty acids and alcohols biodegrade in the environment relatively quickly. If these byproducts go on to find a new use elsewhere, that’s wonderful, but it also has an end of life, which means it won’t accumulate in the environment as plastics have,” said Sadow.
###
The research is further discussed in the paper, “Catalytic Carbon-Carbon Bond Cleavage and Carbon-Element Bond Formation Gives New Life for Polyolefins as Biodegradable Surfactants,” authored by Uddhav Kanbur, Guiyan Zang, Alexander L. Paterson, Puranjan Chatterjee, Ryan A. Hackler, Massimiliano Delferro, Igor I. Slowing, Fréderic A. Perras, Pingping Sun, Aaron D. Sadow; and published in Chem.
The research was conducted by the Institute for Cooperative Upcycling of Plastics (iCOUP), led by Ames Laboratory. iCOUP is an Energy Frontier Research Center consisting of scientists from Ames Laboratory, Argonne National Laboratory, UC Santa Barbara, University of South Carolina, Cornell University, Northwestern University, and the University of Illinois Urbana-Champaign.
Ames Laboratory is a U.S. Department of Energy Office of Science national laboratory operated by Iowa State University. Ames Laboratory creates innovative materials, technologies and energy solutions. We use our expertise, unique capabilities and interdisciplinary collaborations to solve global problems. DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
Media Contact
Laura Millsaps
[email protected]
Original Source
https:/




