Researchers at Children’s National Health System examine the link between the phthalate MEHP and cardiac arrhythmias
Credit: Circulation: Arrhythmia and Electrophysiology, Plasticizer Interaction with the Heart, Volume: 12, Issue: 7, DOI: (10.1161/CIRCEP.119.007294)
Calling an ambulance during an emergency, emailing a breaking news or journal article before a 5 p.m. deadline and maintaining conditions during the fifth week of a 6-week lab study, without altering the light or temperature, requires electricity and translates into time, money and lives. During critical moments, we appreciate the tiny particles and ions in electric currents that power our phones, computers or laboratory equipment. We seldom think about the speed of these connections or potential disruptors when conditions are stable. The same applies to the electric currents, or electrophysiology, of our heart.
Arrhythmias, irregular heart rhythms, affect millions of Americans but can be controlled with routine screenings and preventive care. In critical settings, such as an ICU, doctors monitor arrhythmias, which range from a patient’s heart beating too slow to too fast. Helping a patient maintain a steady heart rate, especially if they are at risk for cardiac complications, may support a faster recovery, shorter hospital stays, reduced health care costs and improved health outcomes, such as avoiding complications from heart failure or stroke.
A preclinical study, entitled “Plasticizer Interaction With the Heart,” appears in the July issue of Circulation: Arrhythmia and Electrophysiology and examines the role plastic exposure, akin to exposure in a medical setting, has on heart rhythm disruptions and arrhythmias.
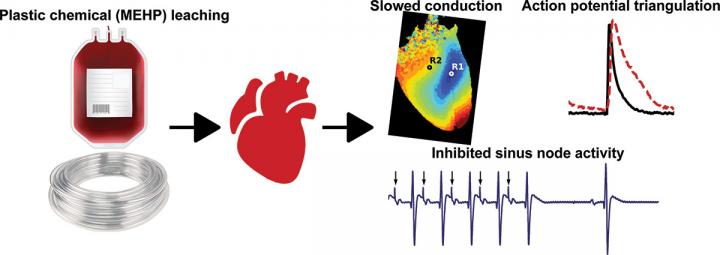
The research team, led by researchers at Children’s National Health System, discovered increased risks for irregular heart rhythms after exposing intact, in vitro heart models to 30 minutes of mono-2-ethylhexyl phthalate (MEHP), a metabolite from Di-2-ethylexyl phthalate (DEHP). DEHP is a chemical commonly used to make plastics pliable in FDA-approved medical devices. This phthalate accounts for 40% of the weight of blood storage bags and up to 80% of the weight of tubes used in an intensive care setting, such as for assisted feeding or breathing, and for catheters used in diagnostics or to conduct minimally invasive cardiac procedures.
The team chose to study the heart’s reaction to 60 μM of MEHP, a level comparable to stored blood levels of MEHP observed in pediatric patients and in neonatal exchange transfusion procedures. They found 30-minute exposure to MEHP slowed atrioventricular conduction and increased the atrioventricular node effective refractory period. MEHP prolonged action potential duration time, enhanced action potential triangulation, increased the ventricular effective refractory period and slowed epicardial conduction velocity, which may be due to the inhibition of Nav 1.5, or sodium current.
“We chose to study the impact of MEHP exposure on cardiac electrophysiology at concentrations that are observed in an intensive care setting, since plastic medical products are known to leach these chemicals into a patient’s bloodstream,” says Nikki Gillum Posnack, Ph.D., a principal investigator with the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National and an assistant professor of pediatrics at the George Washington University School of Medicine and Health Sciences. “In critical conditions, a patient may have a blood transfusion, require extracorporeal membrane oxygenation, undergo cardiopulmonary bypass or require dialysis or intravenous fluid administration. All of these scenarios can lead to plastic chemical exposure. Our research team wants to investigate how these plastic chemicals can impact cardiac health.”
In this review, Dr. Posnack’s team mentions one reason for the observed changes in the preclinical heart models may be due to the structure of phthalates, which resemble hormones and can interfere with a variety of biological processes. Due to their low molecular weight, these chemicals can interact directly with ion channels, nuclear receptors and other cellular targets.
Existing epidemiological research shows associations between exposure to phthalates and adverse health outcomes, including metabolic disturbances, reproductive disorders, inflammatory conditions, neurological disorders and cardiovascular disease. This is the first study to examine the link between cardiac electrophysiology in intact hearts and exposure to MEHP, comparable to levels observed in an ICU.
Dr. Posnack’s team previously found DEHP reduced cellular electrical coupling in cardiomyocyte cell models, which slowed conduction velocity and produced an arrhythmogenic phenotype. A microarray analysis found heart cells treated with DEHP led to mRNA changes in genes responsible for contracting and calcium handling. Another preclinical study showed DEHP altered nervous system regulation of the cardiovascular system. Future studies to expand on this research may include the use of larger preclinical models or human assessments. For the latter, stem cell-derived cardiomyocytes can be used to compare the safety profile of plastic chemicals with potential alternatives.
An accompanying editorial, entitled “Shocking Aspects of Nonconductive Plastics,” authored by cardiology researchers at the University of Wisconsin-Madison, puts this novel research into perspective. Like Dr. Posnack, the team notes that while the clinical impact plasticizers have on heart health still needs to be determined, the work contributes to compelling data among multiple researchers and shows DEHP and MEHP are not inert substances.
“Toxic plasticizers in children’s toys and baby products hit public headlines 20 years ago, but exposure to these compounds is up to 25x higher in patients undergoing complex medical procedures,” write the University of Wisconsin-Madison researchers. “We readily (and unknowingly) administer these compounds, and at times in high quantity, to some of our most vulnerable patients. This work highlights the need for further investigation into short and long-term plasticizer exposure on cardiac electrophysiology.”
The Agency for Toxic Substances and Disease Registry (ATSDR), part of the Centers for Disease Control and Prevention (CDC), released a public health statement about DEHP in 2002, noting more research in humans is needed to issue formal warnings against this phthalate.
ATSDR states there is no conclusive evidence about the adverse health effects of children exposed to DEHP in a medical setting, such as procedures that require the use of flexible tubing to administer intravenous fluids or medication. However, the CDC statement includes limits of DEHP exposure, based on preclinical models, used to guide upper DEHP limits in consumer products, including food packaging, drinking water, and air quality in the workplace.
“It’s important to note that this was a preliminary study performed on an ex vivo model that is largely resilient to arrhythmias”, says Rafael Jaimes III, Ph.D., the first author of the study and a senior scientist at Children’s National. “Due to the nature of the design, it was somewhat alarming that we found such significant effects. I predict that electrophysiological disturbances will be more pronounced in models that more closely resemble humans. These types of models should absolutely be studied.”
“And, importantly, our results may incentivize the development and use of new products that are manufactured without phthalates,” Dr. Posnack adds.
These questions are powering Dr. Posnack and her team through a decade-long, multi-institution research investigation to understand how plastic chemicals and medical device biomaterials can impact cardiac health.
###
Additional study authors for this paper include Damon McCullough, B.S., Bryan Siegel, M.D., Luther Swift, Ph.D., Daniel McInerney, B.S., and James Hiebert, B.S., with the Sheikh Zayed Institute for Pediatric Surgical Innovation and Children’s National Heart Institute, part of Children’s National Health System, in Washington, D.C.; Erick A. Perez-Alday, Ph.D., and Larisa G Tereshchenko, M.D., Ph.D., with the Knight Cardiovascular Institute at Oregon Health and Science University in Portland, Ore.; Javier Saiz, Ph.D., and Beatriz Trenor, Ph.D., with Ci2B-Universitat Politecnica de Valencia in Spain and Jiansong Sheng, Ph.D., from CiPA Lab, LLC, in Rockville, Md.
The study was supported by the National Institutes of Health (R00ES023477 and R01HL139472), Children’s Research Institute and Children’s National Heart Institute. NVIDIA corporation provided graphics processing, with partial support by the Direccion General de Politica Cientifica de la Generalitat Valenciana (PROMETEU2016/088).
To interview Dr. Posnack or a study author, please contact Jessica Frost at 301-828-7521 or Beth Riggs at [email protected] or 301-244-6713.
About Children’s National
Children’s National Health System, based in Washington, D.C., has served the nation’s children since 1870. Children’s National is the nation’s No. 6 pediatric hospital and, for the third straight year, is ranked No. 1 in newborn care, as well as ranked in all specialties evaluated by U.S. News & World Report. It has been designated two times as a Magnet hospital, a designation given to hospitals that demonstrate the highest standards of nursing and patient care delivery. This pediatric academic health system offers expert care through a convenient, community-based primary care network and specialty outpatient centers in the D.C. Metropolitan area, including the Maryland suburbs and Northern Virginia. Home to the Children’s Research Institute and the Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s National is the seventh-highest NIH-funded children’s hospital in the nation. Children’s National is recognized for its expertise and innovation in pediatric care and as a strong voice for children through advocacy at the local, regional and national levels.
For more information, follow Children’s National on Facebook and Twitter.
Media Contact
Jessica Frost
[email protected]
Related Journal Article
http://dx.




