A groundbreaking breakthrough in the realm of cancer radiotherapy has emerged from the laboratories of The University of Osaka, Japan, promising to redefine the future landscape of oncological treatments. Researchers have uncovered specific conditions that enable carbon ion beams—when delivered at ultra-high dose rates—to significantly protect normal cells from radiation damage. This phenomenon, known as the FLASH effect, could herald a new era of cancer care, mitigating collateral damage often inflicted upon healthy tissues during radiation therapy and ultimately enhancing patient outcomes and quality of life.
Radiation therapy remains a cornerstone in the fight against cancer, utilized in efforts to eradicate tumor cells with high precision. However, a perennial challenge in this modality has been the unavoidable exposure of surrounding healthy cells to radiation, leading to often debilitating side effects. The FLASH effect, first identified in 2014, describes a remarkable biological response wherein radiation doses administered at rates exceeding 40 Gray per second (Gy/s) can drastically minimize damage to normal tissues while retaining the ability to target malignant cells effectively.
While early investigations into the FLASH effect predominantly focused on photon (X-ray), electron, and proton radiation modalities, translating this phenomenon to carbon ion therapy—known for its superior targeting accuracy and potent biological lethality—had remained elusive. Carbon ion beams deliver a high linear energy transfer (LET), making them especially effective at inducing irreparable DNA damage within cancer cells. Yet, reproducing the FLASH effect with carbon ions has been technically challenging due to complexities in generating and controlling ultra-high dose rates, as well as understanding the interplay between dose rate, LET, and cellular oxygen levels.
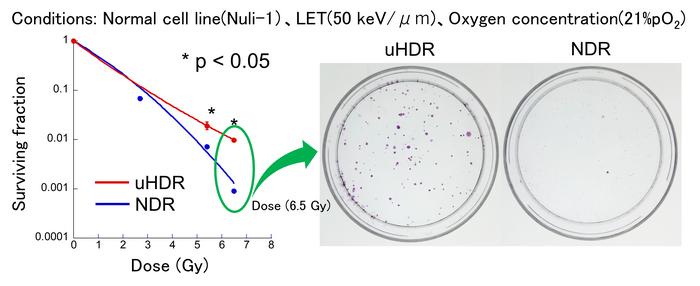
The interdisciplinary team at Osaka tackled these challenges using a bespoke synchrotron system at the Osaka Heavy Ion Therapy Center, meticulously varying parameters such as oxygen concentration and LET to illuminate their influence on cell survival. Employing three human cell lines—two representing normal tissue and one cancerous—the researchers observed a pronounced increase in viability of normal cells when exposed to carbon ion beams at ultra-high dose rates compared to conventional dose rates. This cell-sparing effect was clearly evident even under normoxic conditions, overturning previous assumptions that low oxygen tensions were necessary to trigger FLASH-related benefits.
Moreover, the cell sparing phenomenon was most significant at LET values around 50 keV/μm, a typical condition near the Bragg peak region where carbon ions deposit maximum energy during therapy. This specificity suggests an intricate dependence on the interplay between energy deposition patterns and biological responses, implicating LET as a pivotal parameter in optimizing carbon ion FLASH protocols. Complementary assays revealed reduced markers of DNA double-strand breaks and other molecular indicators of radiation-induced damage, pointing to altered damage mechanisms or enhanced repair pathways under ultra-high dose rate exposure.
Lead author Kazumasa Minami emphasized the novelty of these insights, stating that this represents the first rigorous demonstration of a FLASH effect with carbon ions in environments simulating normal physiological oxygenation. Corresponding author Masashi Yagi highlighted the technical originality and the potential translational impact of the findings, noting that “these results open new avenues for the design of carbon ion radiotherapy regimens that maximize tumor control while preserving healthy tissue integrity.”
This advancement not only confirms the existence of FLASH-like benefits in carbon ion therapy but also delineates critical irradiation conditions necessary to harness this effect. The implications for clinical radiotherapy are profound: practitioners could calibrate treatments by fine-tuning dose rate, LET, and oxygenation conditions to exploit the therapeutic window fully. Such precision could mitigate the dose-limiting toxicities that currently restrict radiation doses, enabling stronger, more effective treatments with fewer adverse effects.
The study also paves the way for more extensive explorations into the biological underpinnings of the FLASH effect with high LET radiation. Investigations into immune modulation, tumor microenvironment alterations, and the dynamics of cellular repair networks are anticipated to deepen our understanding and expand the applicability of this phenomenon in diverse cancer types. Integration of ultra-high dose rate carbon ion therapy into clinical trials may consequently redefine standards and improve survival rates globally.
Given the sophistication of generating ultra-high dose rate carbon ion beams, the Osaka team’s success depended on state-of-the-art technology and meticulous experimental design. Their approach combined advanced accelerator physics, cellular radiobiology, and quantitative molecular diagnostics, exemplifying the multidisciplinary effort required to capture and harness this emerging therapeutic effect. The deployment of such high precision and highly controlled irradiation setups sets a new benchmark for future preclinical and clinical studies in FLASH radiotherapy.
Beyond the immediate clinical significance, this research revitalizes interest in the nuanced relationship between radiation qualities and biological outcomes. It challenges long-standing paradigms by revealing that not only total dose but the temporal delivery dynamics and microenvironmental context are vital determinants of treatment efficacy and safety. Understanding and applying such principles are pivotal for the continued evolution of radiotherapy technologies.
This landmark publication, titled “The Appropriate Conditions for the Cell Sparing (FLASH) Effect Exist in Ultra‐high Dose Rate Carbon Ion Irradiation,” was reported in the journal Anticancer Research. It marks a milestone that bridges a critical knowledge gap and propels carbon ion therapy to the forefront of innovative cancer treatment strategies.
As the scientific community grapples with translating laboratory findings to clinical practice, the Osaka study provides a compelling foundation and clear roadmap for future research. Its findings invigorate hopes of harnessing the FLASH effect across various ion species, potentially unlocking a universal strategy to minimize collateral damage in radiation oncology.
Ultimately, this discovery embodies a transformative stride toward personalized, precision radiotherapy—an approach that could significantly reduce the burden of treatment-related toxicities, enhance tumor control rates, and improve the overall therapeutic ratio for cancer patients worldwide.
Subject of Research: Cells
Article Title: The Appropriate Conditions for the Cell Sparing (FLASH) Effect Exist in Ultra‐high Dose Rate Carbon Ion Irradiation
News Publication Date: 5-Mar-2025
References: DOI: 10.21873/anticanres.17483
Image Credits: Masashi Yagi
Keywords: Radiation therapy, Cancer treatments, Medical treatments
Tags: biological response to radiationcancer treatment breakthroughscarbon ion synchrotron technologycollateral damage in oncological treatmentsenhancing patient outcomes in cancer careFLASH effect in cancer therapyminimizing side effects of cancer treatmentnormal cell protection in radiation therapyradiation therapy advancementstargeted radiation therapy innovationsultra-high dose rate radiationUniversity of Osaka cancer research