By polarizing different cell fate determinants at opposite cell poles, asymmetric cell division (ACD) that produces distinct daughter cells is an evolutionarily conserved mechanism to generate cellular diversity in both eukaryotes and prokaryotes.
Credit: SIAT
By polarizing different cell fate determinants at opposite cell poles, asymmetric cell division (ACD) that produces distinct daughter cells is an evolutionarily conserved mechanism to generate cellular diversity in both eukaryotes and prokaryotes.
The polarization of the scaffold-signaling hubs at cell poles constitutes the basis of ACD. However, the biomolecular basis and regulatory mechanism of the polar signaling complexes has been largely unclear.
Recently, a research team led by Prof. ZHAO Wei from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences proposed that the polar organelle development scaffold protein PodJ in the new cell pole forms biomolecular condensates with physiological functions via phase separation, which help to establish and regulate the asymmetry of bacterial cells.
The study was published in Nature Communications on Nov. 23.
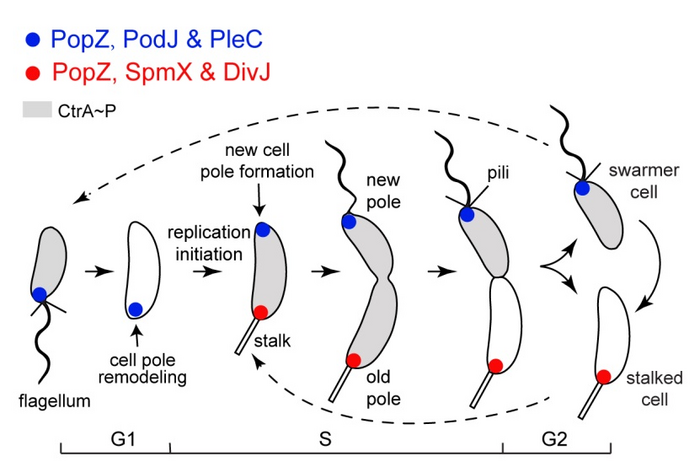
As a well-established model for studying bacterial ACD, Caulobacter crescentus produces a motile swarmer cell and a sessile stalked cell during each cell cycle. In the pre-division cell stage, the polarization of new-pole signaling proteins by the scaffold PodJ coordinates to modulate the phosphorylation levels of a set of downstream signaling proteins, thus determining the cell fate of C. crescentus.
The researchers found that phase separation played an essential role in the C. crescentus PodJ-signaling hub assembly. PodJ formed biomolecular condensates both in vitro and in vivo. Either the coiled-coil 4–6 region or the intrinsically disordered region in PodJ could mediate PodJ phase separation. In addition, both of the phase separation-related domains were found to be involved in recruiting client signaling proteins, indicating that phase separation of PodJ functionally contributes to forming the PodJ–client complexes.
Moreover, a negative regulation of PodJ phase separation by the old-cell-pole scaffold protein SpmX was observed. SpmX inhibited PodJ condensate formation and promoted PodJ condensate aging in vitro. In living cells, SpmX was found to impede the cell-pole accumulation of PodJ and client recruitment, suggesting it may be involved in the new-to-old cell-pole remodeling.
“The results revealed that phase separation modulates the assembly and dynamics of scaffold-signaling hubs in C. crescentus,” said Prof. ZHAO Wei, corresponding author of the study. “It may serve as a general biophysical approach for assembling scaffold-signaling complexes and regulating ACD. Similar methods could be employed for rational engineering of artificial organelles and other membraneless biocatalytic compartments.”
Journal
Nature Communications
DOI
10.1038/s41467-022-35000-2
Article Title
Phase separation modulates the assembly and dynamics of a polarity-related scaffold-signaling hub
Article Publication Date
23-Nov-2022




