Credit: Bryan Welm
WASHINGTON, January 21, 2021 — Cancer is a major, worldwide challenge, and its impact is projected to escalate due to aging and growth of the population. Researchers recognize that new approaches to diagnose and treat deadly cancers, including identifying new drugs to treat cancer, will be essential to curbing the growing impact of the disease.
While decades of investment in research have resulted in substantial improvements in surviving cancer, a key challenge remains in identifying new drugs that improve outcomes for cancer patients, particularly for cancers when tumors have spread throughout the body.
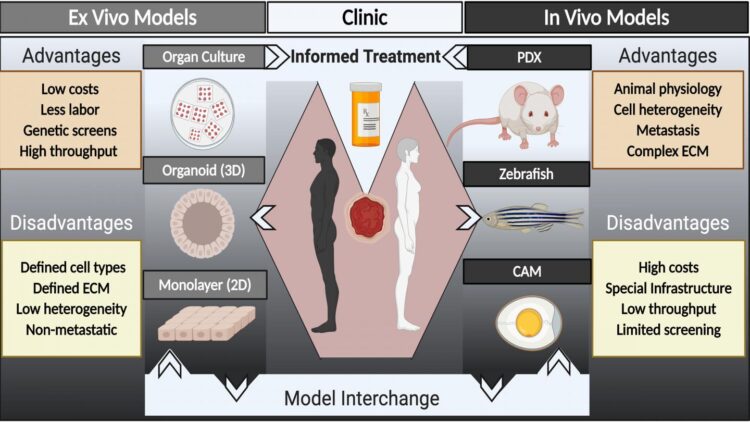
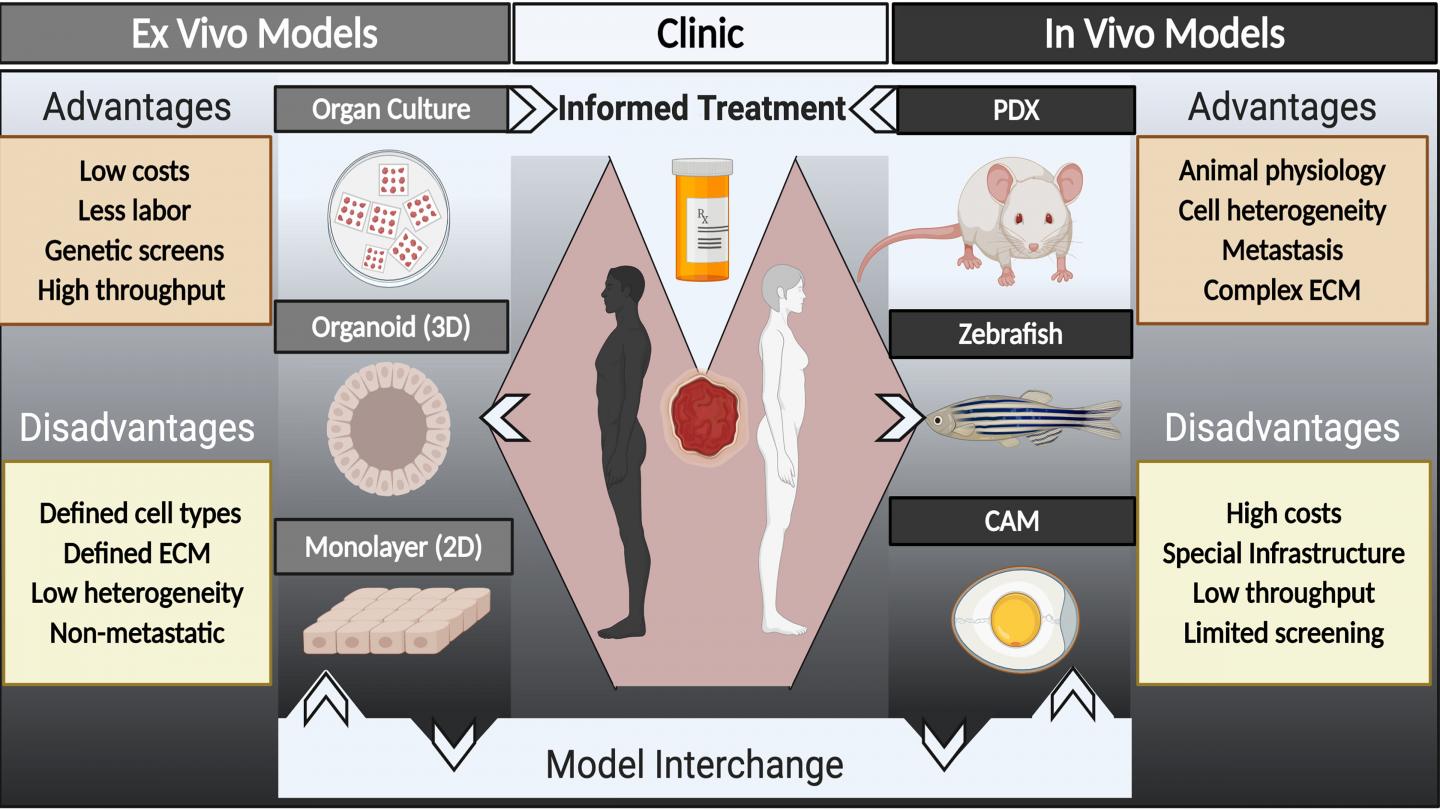
In APL Bioengineering, by AIP Publishing, researchers suggest a major hurdle to identifying new drugs is the paucity of models — organisms mimicking human cancers in a lab setting — for cancer research that accurately represent patient tumors. They provide a perspective on strategies for developing models to inform cancer treatment using models from individual patients and where the field needs to go in terms of research in animal systems and in culture systems.
“In addition to having better models for cancer research, we are trying to evolve patient-derived models to the point where we can have speedy and reliable drug testing on patient specimens to help personalize cancer care,” said author Alana Welm. “This is the concept of functional precision oncology.”
In functional precision oncology, tumor samples from individual patients are tested for susceptibility to various drugs in the context of a patient-derived xenograft or culture system in order to guide patient therapy during the course of their disease.
The researchers suggest an even more powerful approach to accelerating the pace of cancer research would be to couple patient-derived model development with the plethora of clinical trials being run every day.
If clinical data and models were collated and shared along with drug response information, machine learning could facilitate analysis of this big data to discover complex patterns of drug response or resistance across individuals, which could then be further tested in the patient-derived models.
The researchers envision that patients’ tumors may be bioinformatically profiled to identify a complex set of features that can be used to predict response to various therapies and informed by functional drug response data collected from previous studies. The researchers believe this would facilitate selection of more effective drugs earlier in treatment, while preventing administration of toxic drugs that offer no benefit.
These types of data could even be integrated with germline DNA sequence variants that predict aberrant drug metabolism and toxicity for an even more personalized approach to reduce mortality from cancer while simultaneously reducing toxicity as much as possible.
###
The article “Towards improved models of human cancer” is authored by Bryan E. Welm, Christos Vaklavas, and Alana L. Welm. The article appears in APL Bioengineering (DOI: 10.1063/5.0030534) and can be accessed at https:/
See also: Combining best of both worlds for cancer modeling at https:/
ABOUT THE JOURNAL
APL Bioengineering is an open access journal publishing significant discoveries specific to the understanding and advancement of physics and engineering of biological systems. See http://aip.
Media Contact
Larry Frum
[email protected]
Related Journal Article
http://dx.