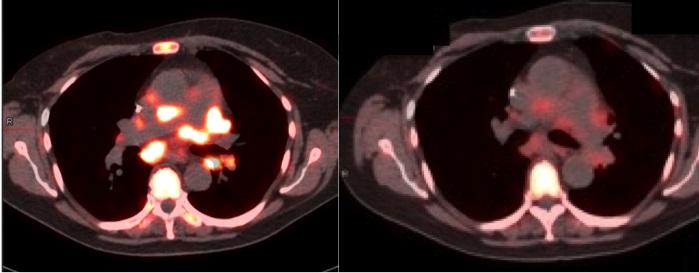
Credit: Penn Medicine
PHILADELPHIA – New molecular imaging technologies can make it easier to diagnose, monitor, and treat cancers while potentially saving patients from undergoing therapies that are likely to be ineffective and playing a role in minimizing side effects, according to experts from the Abramson Cancer Center and the Perelman School of Medicine at the University of Pennsylvania. In a review published online today in JAMA Oncology, the Penn team says finding a way to use these techniques more widely in clinical settings should be a top priority.
Precision cancer care focuses on identifying the specific biomarkers of a patient's cancer, which can help doctors make decisions about the best treatment options. A traditional way to learn about the genetic makeup of cancer is through a biopsy – in which doctors have to physically remove tissue from a patient and then examine it. But new molecular imaging, which can be used to complement the biopsy and is noninvasive, can provide added benefit in certain cases, especially when multiple examinations are needed.
There are four main areas where molecular imaging can have a major impact, according to the study's lead author David A. Mankoff, MD, PhD, the Gerd Muehllehner Professor of Radiology and director of the PET Center at the Perelman School of Medicine at the University of Pennsylvania. First, it can help identify patients most likely to benefit from targeted therapy.
"Once we start treatment, it can also help us plan radiotherapy treatment and help define the boundaries of the active tumor," Mankoff said.
Second, it can monitor the movement of drugs throughout the body to guide drug dosing and minimize side effects. Similarly, it can also monitor whether those drugs are having an effect. Finally, all of this data can be combined to predict patient outcomes including overall survival.
Unlike X-ray and ordinary magnetic resonance imaging (MRI), which reveal the large-scale structures of tissues in the body, molecular imaging uses special imaging "probe" compounds–injected into the patient–to highlight a desired molecular target in a tissue of interest. FDG-PET, one of the only molecular imaging techniques routinely used in oncology, employs a glucose-like probe, FDG, with a radioactive isotope of fluorine attached as a beacon. Once it is injected into the bloodstream, the FDG probe quickly accumulates in tumors, which tend to make heavy use of glucose. Thus, it "lights up" those tumors on a PET (positron emission tomography) scan.
FDG-PET, a method that the University of Pennsylvania helped pioneer, has been used for more than two decades to detect tumors and determine the extent to which cancer has spread. But the newer PET probes now in development and testing are meant for many other applications in cancer medicine.
Two new classes of probe that show particular promise are designed to bind to estrogen and HER2 receptors. Breast, uterine, and ovarian tumors often use these receptors to boost their growth, and many cancer drugs target them. Detecting the presence of tumor estrogen or HER2 receptors with PET scans would enable oncologists to examine all sites of cancer for each patient, choose the appropriate drug treatment more quickly, monitor the tumor for changes that would necessitate a switch to another treatment, and even evaluate how well a drug is hitting its receptor targets.
Imaging with the new PET probes also could reveal receptors or other tumor-related markers at sites where the cancer may have spread, including bone, which is much harder to biopsy.
The probes targeted to breast cancer have shown great promise so far in breast cancer clinical trials, and Mankoff and his Penn Medicine colleagues have helped lead this research effort. One national, multi-center trial focused on the estrogen receptor is co-chaired by oncologist Amy Clark, MD, MSCE, a co-author of the review and an assistant professor of medicine at the Abramson Cancer Center at the University of Pennsylvania. Another smaller trial of estrogen receptor imaging is underway at the Abramson Cancer Center's 2-PREVENT Translational Center of Excellence.
"Many of these methods are already being studied in clinical trials, but the path from clinical trials to routine clinical use is seldom easy," Mankoff said. "And molecular imaging methods face some particularly challenging hurdles such as the need to deliver the short-lived imaging probes to centers performing the imaging."
Because these methods are so new to oncologists, there is no standard procedure for testing them, or for making an empirical case for their clinical value to the FDA, medical insurers, and ultimately oncologists themselves. "We don't have a good framework yet for moving these potentially powerful diagnostic tools into routine clinical use," Mankoff said. "Among other things, we need to bring the imaging and oncology communities together to find the best way forward."
Mankoff and his colleagues argue that making a strong clinical case for these new imaging techniques will mean demonstrating their ability to improve traditional treatment outcomes such as progression-free survival and quality of life. Clinical trials of these methods also could take into account the value of avoiding ineffective treatments. In one recent trial, researchers showed that a combination of FDG and HER2-targeted PET imaging was 100 percent accurate in predicting patient responses to a costly new anti-HER2 breast cancer drug.
"In that case, molecular imaging could have directed treatment to patients highly likely to benefit and spared many other patients the toxic effects and costs of ineffective therapy," Mankoff said.
Above all, Mankoff says testing of new imaging methods should focus on applications where they clearly represent an advance for patients over other imaging or biopsy-based techniques.
"These clinical trial results for the new molecular imaging methods are going to be compelling for patients and their referring oncologists only when they address clinical challenges not met by existing approaches," he said.
Michael D. Farwell, MD, an assistant professor of Radiology at Penn, and Daniel A. Pryma, MD, associate professor of Radiology at Penn, also co-authored the review.
###
Penn Medicine is one of the world's leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation's first medical school) and the University of Pennsylvania Health System, which together form a $5.3 billion enterprise.
The Perelman School of Medicine has been ranked among the top five medical schools in the United States for the past 18 years, according to U.S. News & World Report's survey of research-oriented medical schools. The School is consistently among the nation's top recipients of funding from the National Institutes of Health, with $373 million awarded in the 2015 fiscal year. T
he University of Pennsylvania Health System's patient care facilities include: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center — which are recognized as one of the nation's top "Honor Roll" hospitals by U.S. News & World Report — Chester County Hospital; Lancaster General Health; Penn Wissahickon Hospice; and Pennsylvania Hospital — the nation's first hospital, founded in 1751. Additional affiliated inpatient care facilities and services throughout the Philadelphia region include Chestnut Hill Hospital and Good Shepherd Penn Partners, a partnership between Good Shepherd Rehabilitation Network and Penn Medicine.
Penn Medicine is committed to improving lives and health through a variety of community-based programs and activities. In fiscal year 2015, Penn Medicine provided $253.3 million to benefit our community.
Media Contact
John Infanti
[email protected]
215-301-5221
@PennMedNews
http://www.uphs.upenn.edu/news/
############
Story Source: Materials provided by Scienmag