CORVALLIS, Oregon – An Oregon State University researcher who made color history in 2009 with a vivid blue pigment has developed durable, reddish magentas inspired by lunar mineralogy and ancient Egyptian chemistry.
Credit: Image provided by Mas Subramanian, Oregon State University
CORVALLIS, Oregon – An Oregon State University researcher who made color history in 2009 with a vivid blue pigment has developed durable, reddish magentas inspired by lunar mineralogy and ancient Egyptian chemistry.
Mas Subramanian, distinguished professor of chemistry, and collaborators at OSU report the findings of the study, funded by the National Science Foundation, in the journal Chemistry of Materials.
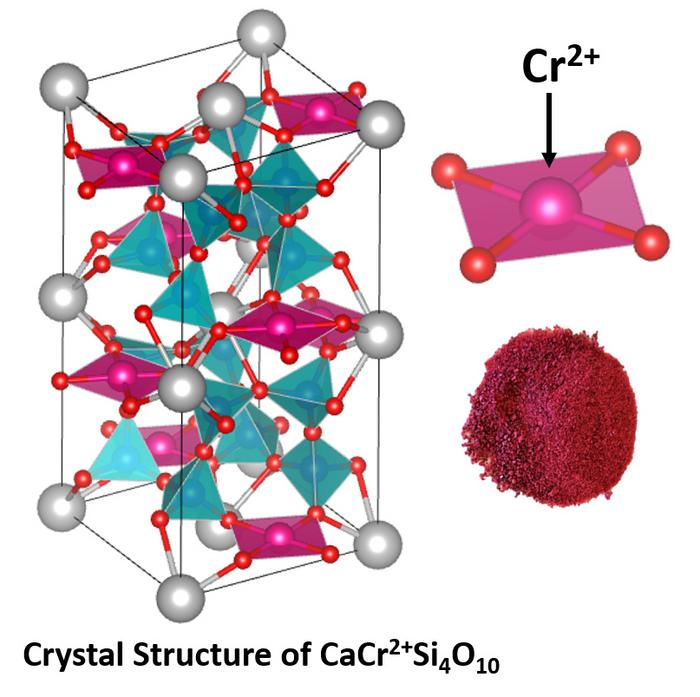
The new pigments, which could be used as energy-efficient coatings for vehicles and buildings, are based on divalent chromium, Cr2+, and are the first to use it as a chromophore; chromophores are the parts of a molecule that determine color by reflecting some wavelengths of light while absorbing others.
“To date, no earth-based mineral has been reported to contain chromium in the divalent state as one of the components,” said Subramanian, the Milton Harris Professor of Materials Science in the OSU College of Science. “However, the analysis of lunar mineral samples collected from Apollo missions showed the occurrence of chromium in the divalent state.”
Divalent chromium has the same number of unpaired electrons as trivalent manganese, the chromophore responsible for the intense color of YInMn blue, which Subramanian’s team discovered 15 years ago. The Shepherd Color Company licensed YInMn blue for use in a wide range of coatings and plastics, and it also inspired a new Crayola crayon color: Bluetiful.
When YInMn blue was discovered, researchers had been experimenting with new materials that could be used in electronics applications and mixed manganese oxide – which is black in color – with other chemicals, then heated them in a furnace to nearly 2,400 degrees Fahrenheit.
One of their samples turned out to be a brilliant blue, named YInMn blue after the component elements yttrium, indium and manganese. It was the first blue pigment discovery in two centuries and a huge advance in safety and durability as well as vividness.
In the new study, Subramanian, research associate Jun Li and graduate student Anjali Verma were inspired by the divalent copper that serves as a chromophore in Egyptian blue, which is the world’s first known synthetic pigment and dates to more than 5,000 years ago.
The researchers replaced the divalent copper in Egyptian blue with divalent chromium, leading to durable, reddish magenta pigments. To stabilize the divalent chromium on Earth, researchers maintained high temperatures, almost 2,500 degrees Fahrenheit, under high vacuum during the synthesis that started from chromium metal, chromium trioxide and other chemicals.
“Most of the magenta-colored pigments used today are organic chemicals and suffer from stability issues when exposed to ultraviolet rays and heat from the sun because they can break down organic chemical bonds,” Subramanian said. “Inorganic magenta pigments are rare, and most require a significant amount of cobalt salts that are hazardous to both humans and the environment.”
The magenta pigments developed by OSU researchers are thermally and chemically inert because of their high preparation temperature and remain unaltered structurally and optically upon exposure to acid and alkali, the authors note.
In addition, unlike pigments that contain cobalt, the chromium-based magenta pigments are highly reflective of heat from the sun – meaning they have a cooling property that would lead to energy savings for cars and structures coated in them.
“Most pigments are discovered by chance,” Subramanian said. “The reason is because the origin of the color of a material depends not only on the chemical composition but also on the intricate arrangement of atoms in the crystal structure. So someone has to make the material first in a laboratory, then study its crystal structure thoroughly to explain the color.”
Despite recent advances in quantum mechanical theories and computational methods, predicting a crystal structure that will produce an intense inorganic pigment of a desired color is still elusive, he added.
“We got lucky the first time with YInMn blue, and now we are coming up with some fundamental chemical and crystal structural design principles to rationally create new pigments,” he said. “Determining the key structural ingredients required for making vivid colors should allow for shorter times between pigment discoveries. Science doesn’t always follow a prescribed path, but we’re exploring pigments with divalent chromium as a chromophore in diverse coordination environments in crystal structures of various inorganic compounds.”
The NSF funding for the just-published study was a special grant earmarked for high-risk, high-reward research. The grant is known by the acronym EAGER, which stands for Early Concepts Grants for Exploratory Research.
Journal
Chemistry of Materials
DOI
10.1021/acs.chemmater.4c00253
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Cr2+ in Square Planar Coordination: Durable and Intense Magenta Pigments Inspired by Lunar Mineralogy
Article Publication Date
11-Apr-2024




