Credit: Katsufuji Laboratory, Waseda University
A new study by researchers from Waseda University and the University of Tokyo found that orbital ordering in a vanadate compound exhibits a clear nucleation-growth behavior.
“We believe that this is the first observation of its kind, where electrons in an inorganic solid created two soft phases similar to vapor and water, and where a nucleation-growth behavior was observed due to the surface tension created between the phases,” says Takuro Katsufuji, professor of physical sciences at Waseda University and principal investigator of this study.
The researchers published their peer-reviewed findings in Nature Communications on May 11, 2020.
When water vapor condenses, the vapor turns into dew as water nuclei form and grow, transitioning phases from gas to liquid. This formation and growth of the nuclei is called the nucleation-growth process, and it occurs in various types of phase transitions.
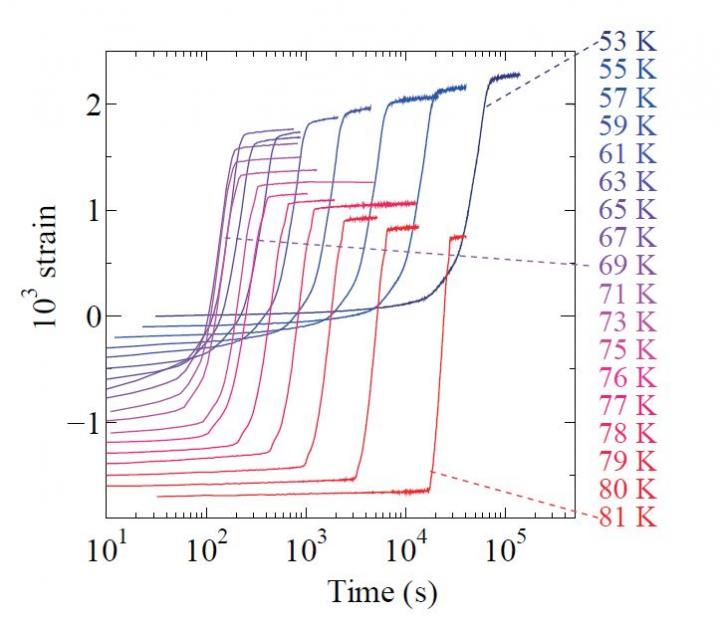
Though phase transitions also occur in solids, a nucleation-growth behavior has never been observed during electron-based phase transitions in inorganic materials. The most likely explanation is because there needs to be surface tension for this behavior to occur, but the hard solids could only create negligible surface tension compared with a bulk elastic energy.
In their study, Katsufuji and his team used the vanadate compound BaV10O15, an oxide with vanadium and barium. Previously, they found that at 130K, or approximately -140°C, the direction of orbitals possessed by the vanadium’s electrons align, triggering a phase transition known as orbital ordering in this compound.
“Knowing this, what we did in our recent study was to partially replace vanadium with titanium, a chemical element located on the left of vanadium on the periodic table, to control the transition temperature of the orbital ordering,” Katsufuji explains. “We revealed that the nucleation-growth process was happening by measuring the time dependence of the electrical resistivity, magnetic susceptibility, and the strain in the orbital ordering of this vanadate, and discovered that the electrons had created two vapor-water like soft phases in this organic solid and that there was surface tension between the two phases.”
In the past, methods to obtain desirable characteristics with two-phase coexistence in solids have been tested with various materials and devices, but in most cases, it has been difficult to control the volume ratio and the forms of the two phases. However, it is expected that for these newly discovered soft phases, controlling the volume ratio and its forms could be done more easily. Also, BaV10O15 is known for its excellent performance as a thermoelectric material that can generate electricity from temperature difference, making this compound with two phases coexisting an attractive material.
Katsufuji adds, “The reason why nucleation-growth process occurs in BaV10O15 is because the surface tension between the two phases is quite large as a result of the coupling of degrees of freedom called orbitals and spins in electrons. We hope to progress research from the perspective that this is a new phenomenon that arises from such coupled degrees of freedom of electrons.”
The researchers plan to measure various physical quantities of the vanadate compound at a state where the two phases coexist and understand how its physical properties can change as well as how its characteristics can improve as a functional material. Further, BaV10O15 is the first material as an inorganic compound where nucleation-growth process was observed, but it will be necessary to find whether there are other materials that exhibit nucleation-growth behavior.
###
Reference
Journal: Nature Communications
Article title: Nucleation and growth of orbital ordering
Authors: Takuro Katsufuji, Tomomasa Kajita, Suguru Yano, Yumiko Katayama, and Kazunori Ueno
DOI: 10.1038/s41467-020-16004-2
About Waseda University
Located in the heart of Tokyo, Waseda University is a leading private research university that has long been dedicated to academic excellence, innovative research, and civic engagement at both the local and global levels since 1882. Today, the student body at Waseda is approximately 50,000, nearly 8,000 of whom are from overseas, hailing from 125 countries. To learn more about Waseda University, visit https:/
Media Contact
Jasper Lam
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




