Researchers have succeeded for the first time in controlling the function of sperm by optogenetics. They inserted a light-activated enzyme for cAMP synthesis into mouse sperm that lacked the endogenous enzyme. Sperm of these mice are usually non-motile, and the mice consequently infertile. After stimulation of these sperm with blue light, they produce cAMP, start to swim again, and are even able to fertilise eggs. Using optogenetics, the scientists are now able to control not only the influx of ions into nerve cells, and thus their activity, but also signalling pathways in other cell types.
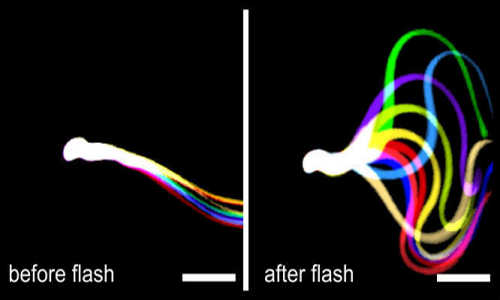
Light stimulation restores flagellar beating. Flagellar waveform of sperm lacking the function SACY enzyme, but containing the light-activated bPAC, before (left) and after stimulation with blue light (right). Successive, aligned, and superimposed images creating a “stop‐motion” image, illustrating one flagellar beating cycle. Scale bar: 30 µm. Photo Credits: Caesar
It is a long-cherished dream of scientists to control cells with light. Light can be switched on and off rapidly and does not interfere with natural cellular processes. The biggest obstacle to realizing this goal was finding a way to furnish cells with “light switches”. In 2002, three German scientists – Peter Hegemann, Ernst Bamberg, and Georg Nagel – proved that the light-sensitive membrane proteins of a unicellular green alga are ion channels and dubbed them channelrhodopsins. Channelrhodopsins can be inserted into cells by genetic engineering methods, making it possible to control cells with light. This discovery established a new research field, known as optogenetics.
Optogenetics has been mainly used to control the electrical activity of nerve cells containing channelrhodopsin by light. Meanwhile, the optogenetic “toolbox” has been enlarged so that it is now possible to switch messenger-mediated signalling pathways in cells on and off. An important cellular messenger is cyclic AMP (cAMP), which controls a wide range of functions such as heart rate, the sense of smell, learning, memory formation, and the fertilisation of eggs. This substance is synthesised by enzymes known as adenylate cyclases.
In 2002, the first light-activated adenylate cyclase (PAC, for photo-activated adenylate cyclase) was discovered. Since then, researchers have encountered more such enzymes. One of the latest prominent examples is bPAC, a light-gated adenylate cyclase from soil bacteria, which was identified by Peter Hegemann at the Humboldt University in Berlin.
In collaboration with Peter Hegemann, scientists at the Center of Advanced European Studies and Research (caesar) in Bonn, headed by Benjamin Kaupp and Dagmar Wachten, generated a genetically modified mouse, whose sperm contain the light-activated adenylate cyclase bPAC. Fertilisation of egg cells is closely linked with cAMP synthesis. Sperm lacking the endogenous adenylate cyclase enzyme are unable to swim: the mice are infertile. However, if the bPAC sperm are stimulated with blue light, the cAMP concentration increases and the sperm swim faster, because the sperm tail beats faster.
However, the researchers’ goal was not only to control the swimming motion with light; they also wanted to use light to regulate fertilization. They therefore inserted bPAC into sperm of a mouse mutant lacking the endogenous enzyme for cAMP production. Sperm of these mice are non-motile, and the male mice are infertile. However, after stimulation with blue light, the sperm began to swim again and were even able to fertilise the egg. It is therefore possible to control something as fundamental as fertilisation with light. Optogenetics has thus conquered another field outside the neurosciences.
Story Source:
The above story is based on materials provided by Max-planck-gesellschaft München.