Credit: ©Science China Press
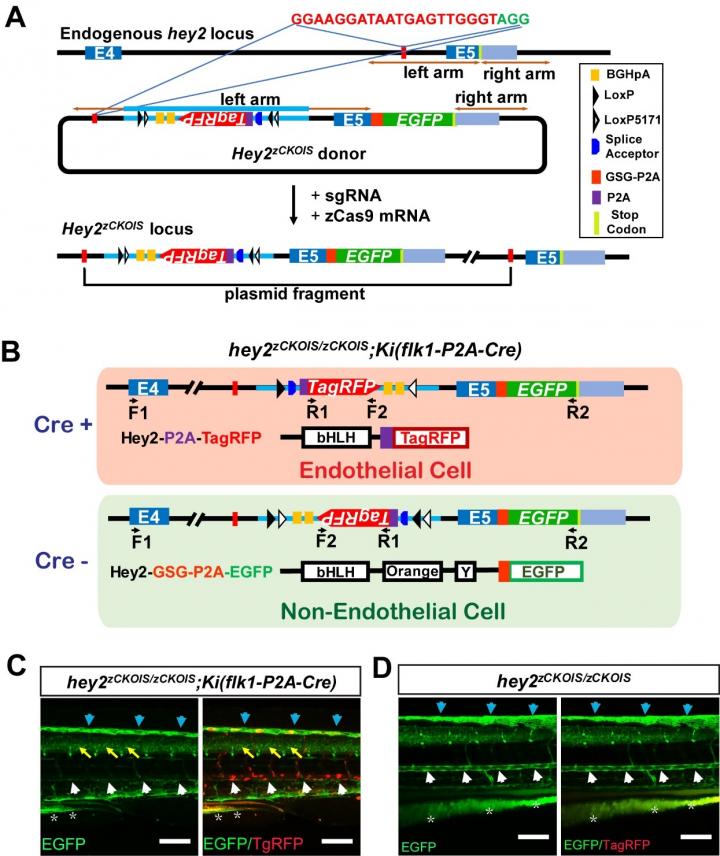
Knockin (KI) animals carrying exogenous sequences integrated at specific genomic loci are invaluable tools for biomedical research. To understand the role of lethal genes in post-embryonic functions, it is usual to use KI animals carrying two loxP insertions at interested genomic loci to generate conditional gene knockout (CKO) animals. The zebrafish (Danio rerio) is a vertebrate animal model excellent for in vivo imaging of biological events. However, to knockin two loxP sites in the zebrafish genome is still a big challenge due to the unavailability of gene targeting techniques for zebrafish embryonic stem cells and the low efficiency of homologous recombination (HR) in fast-developing fertilized zebrafish eggs. Du’s lab previously developed a non-HR-mediated efficient KI strategy without destroying targeted genes, in which a Cas9 target is selected in an intron and the donor plasmid is integrated into the targeted intron. This strategy has been used to efficiently make KI zebrafish and mouse.
“We develop a novel method for making zebrafish CKO and KI switch (zCKOIS) in one step based on our previously reported non-HR-mediated intron targeting. We showed that the zCKOIS cassette can be targeted into the last intron of hey2 with a high efficiency via CRIPSR/Cas9 mediated non-HR insertion.” said Dr Jia Li, the co-first author for this work. A floxed and invertible gene-trap cassette with an RNA slice acceptor is inserted in the intron sequence of the donor plasmid. Without Cre, the zCKOIS cassette will not be inverted and a KI reporter with green fluorescence will be transcripted under the control of the endogenous promoter of the targeted gene. In the present of Cre, the cassette will be inverse, and the targeted gene will be destroyed, associated with the expression of a KO reporter with red fluorescence. “Using this strategy, we generated a hey2zCKOIS fish line and observed green fluorescence (i.e., KI reporter) in various cell types, including glial cells, endothelial cells (ECs) and haematopoietic stem cells (HSCs), similar to the expression pattern of the endogenous hey2 in zebrafish and mouse.” said Dr Jia Li. By injecting Cre mRNA, they validated that the inverted hey2zCKOIS (hey2zCKOIS-inv) coupled with red fluorescence (i.e., KO reporter) is a non-functional KO allele. Finally, they achieved EC-specific KO of hey2 by crossing the hey2zCKOIS with the KI line Ki(flk1-P2A-Cre), in which Cre expression is driven by the EC-specific promoter flk1.
This method realizes the generation of CKO and KI reporter line in one step via efficient non-HR-mediated insertion. The simplification and combination of CKO and KI make the zCKOIS strategy an applicable approach for zebrafish and even other organisms.
###
This work was supported by grants from the Young Scientists Fund of the National Natural Science Foundation of China (Grant No.31500849), Shanghai Municipal Science and Technology Major Project (18JC1410100, 2018SHZDZX05), the Key Research Program of Frontier Sciences (QYZDY-SSW-SMC028), the Strategic Priority Research Program (XDB32010200) of Chinese Academy of Science, the International Partnership Program, Bureau of International Co-operation of Chinese Academy of Science(153D31KYSB20170059), China Wan-Ren Program, and Shanghai Leading Scientist Program.
See the article:
Jia, L., Hongyu, L., Shanye, G, et al. (2019). One-step Generation of Zebrafish Carrying a Conditional Knockout-Knockin Visible Switch via CRISPR/Cas9-Mediated Intron Targeting. Sci China Life Sci, in press, https:/
Media Contact
Du Jiulin
[email protected]
Related Journal Article
http://dx.




