In the evolving landscape of pediatric oncology, osteosarcoma remains a formidable adversary. As the most prevalent primary malignant bone tumor in children and adolescents, its aggressive biology and resistance to conventional treatments pose significant clinical challenges. Recent scientific breakthroughs have shifted the spotlight onto a transcription factor known as Nuclear factor E2-related factor 2 (Nrf2), revealing a complex duality in its function that profoundly impacts chemoradiotherapy resistance in osteosarcoma.
Nrf2 has long been recognized for its critical role in maintaining cellular redox homeostasis. By regulating the expression of antioxidant genes through the antioxidant response element (ARE), Nrf2 protects normal cells from oxidative damage and environmental toxins. However, in the context of malignancy, this protective mechanism takes on a sinister role. In osteosarcoma cells, aberrant activation and overexpression of Nrf2 establish a survival advantage, safeguarding tumor cells from the cytotoxic effects of chemotherapy and radiotherapy.
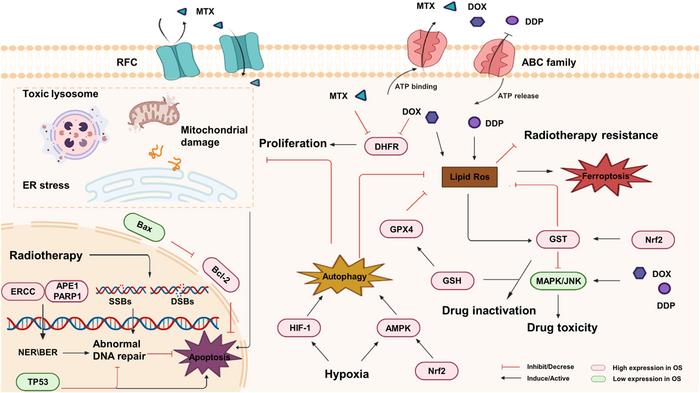
One of the principal ways Nrf2 mediates chemoresistance is through the modulation of intracellular drug concentration. It achieves this by inducing the expression of drug transporter proteins belonging to the ATP-binding cassette (ABC) family and reduced folate carrier (RFC) proteins. These efflux pumps effectively lower the accumulation of chemotherapeutic agents such as cisplatin, doxorubicin, and methotrexate within cancer cells, thereby diminishing drug efficacy. This mechanism is further compounded by Nrf2’s ability to enhance cellular detoxification pathways, enabling malignant cells to neutralize and eliminate harmful compounds more efficiently.
Beyond drug resistance, Nrf2 orchestrates a robust defense against radiotherapy-induced oxidative stress. Ionizing radiation primarily exerts its tumoricidal effect by generating reactive oxygen species (ROS), which inflict DNA damage and promote cell death. Elevated Nrf2 activity in osteosarcoma cells boosts the production of antioxidant enzymes and DNA repair machinery, allowing these cells to swiftly mitigate ROS damage and repair lethal DNA double-strand breaks. This concerted response not only preserves tumor cell viability but also fosters radioresistance, undermining clinical treatment outcomes.
The intricate regulatory network surrounding Nrf2 involves critical signaling pathways that contribute to osteosarcoma pathophysiology. The Keap1-Nrf2-ARE axis, a well-characterized molecular sensor of oxidative stress, becomes dysfunctional in resistant tumors, leading to persistent Nrf2 activation. Meanwhile, crosstalk with the PI3K/AKT pathway amplifies survival signals and promotes metabolic remodeling, further supporting malignant progression. Autophagy, a cellular recycling process, is also modulated by Nrf2, balancing tumor cell survival and death while influencing susceptibility to therapy. Moreover, emerging evidence highlights interactions between Nrf2 and regulated cell death mechanisms like ferroptosis, underscoring its role not only as a protector but also as a modulator of tumor fate decisions.
Crucially, Nrf2’s influence extends beyond survival pathways to govern tumor proliferation and metastasis. Osteosarcoma cells with upregulated Nrf2 demonstrate metabolic reprogramming favoring anabolic growth and adaption to nutrient-deprived microenvironments. This metabolic flexibility, combined with Nrf2’s promotion of epithelial-to-mesenchymal transition (EMT), facilitates tumor dissemination and secondary colonization. Clinical data correlates high Nrf2 expression levels with poorer patient prognoses and reduced overall survival, firmly establishing its oncogenic potential.
Targeting Nrf2 therapeutically is a daunting endeavor due to its indispensable role in protecting normal tissues from oxidative injury. Nonetheless, the discovery of selective inhibitors such as ML385, which disrupt the Nrf2 pathway, offers a promising avenue to sensitize osteosarcoma cells to conventional therapies. Additionally, natural compounds like oridonin have exhibited efficacy in preclinical models by dampening Nrf2-mediated resistance mechanisms and inducing tumor regression. These approaches symbolize the pioneering efforts to exploit tumor-specific vulnerabilities while minimizing collateral damage.
The quest to unravel the multilayered functions of Nrf2 underscores a paradigm shift in understanding osteosarcoma biology. It invites a nuanced appreciation that while antioxidant defense is vital for physiological homeostasis, its hijacking by cancer cells represents a formidable barrier to treatment success. As research intensifies, focusing on the molecular intricacies of Nrf2 regulation and interaction networks may unlock innovative therapeutic strategies tailored for resistant osteosarcoma subtypes.
Further investigations into the role of Nrf2 in modulating autophagy, ferroptosis, and metabolic plasticity will be critical. These studies promise to delineate the balance between tumor suppression and promotion exerted by Nrf2, guiding the design of combination therapies that strategically disrupt survival pathways. Meanwhile, advancements in drug delivery systems and precision medicine hold potential to translate Nrf2-targeted interventions from bench to bedside.
Ultimately, these insights herald a new era in combating osteosarcoma. By deciphering the molecular underpinnings driving chemoradiotherapy resistance, the scientific community paves the way for more effective and personalized treatment regimens. Harnessing the knowledge of Nrf2’s double-edged impact may revolutionize clinical outcomes for pediatric patients battling this aggressive malignancy, offering hope for improved survival and quality of life.
—
Subject of Research: Chemoradiotherapy resistance mechanisms in osteosarcoma focused on the role of Nuclear factor E2-related factor 2 (Nrf2).
Article Title: Nrf2: A key regulator in chemoradiotherapy resistance of osteosarcoma
News Publication Date: 2024
Web References:
http://dx.doi.org/10.1016/j.gendis.2024.101335
References:
Xianglin Peng, Jing Feng, Han Yang, Ping Xia, Feifei Pu, Nrf2: A key regulator in chemoradiotherapy resistance of osteosarcoma, Genes & Diseases, Volume 12, Issue 4, 2025, 101335.
Image Credits: Genes & Diseases
Keywords: Osteosarcoma, Nrf2, chemoradiotherapy resistance, antioxidant response element, drug efflux, DNA repair, reactive oxygen species, Keap1-Nrf2-ARE pathway, PI3K/AKT signaling, autophagy, ferroptosis, tumor proliferation, metastasis
Tags: antioxidant response element regulationATP-binding cassette transporterscellular redox homeostasis in cancerchemoradiotherapy resistance mechanismschemotherapy and radiotherapy in osteosarcomadrug transporter proteins in cancerNrf2 in osteosarcoma treatment resistancenuclear factor E2-related factor 2overcoming osteosarcoma treatment resistance.oxidative damage in malignant tumorspediatric oncology challengessurvival advantage of tumor cells