Credit: Osaka University
Osaka – Antibodies are a powerful weapon system in defending our body against invaders such as bacteria and viruses. Each antibody consists of four polypeptide chains: two heavy chains and two light chains joined to form a Y-shaped molecule. Antibodies recognize a specific antigen unique to its target as they possess the antigen-binding sites located at the upper tips of the Y. While antibody-based therapeutics have been established as front-line drugs, little headway has been made in the use of antibodies as research tools in small molecule drug discovery, particularly in the field of x-ray crystallography.
X-ray crystallography is a technique that uses x-ray diffraction patterns to determine high-resolution, three-dimensional structures of molecules such as proteins, small organic molecules, and materials. The major challenge in X-ray crystallography approaches remains the production of high-quality diffracting crystals.
In recent years, there is increasing use of antibody fragments as crystallization chaperones to aid the structural determination of otherwise "uncrystallizable" or "undruggable" target proteins. The basis for the strategy is to increase the probability of obtaining well-ordered crystals by minimizing the conformational heterogeneity in the target protein.
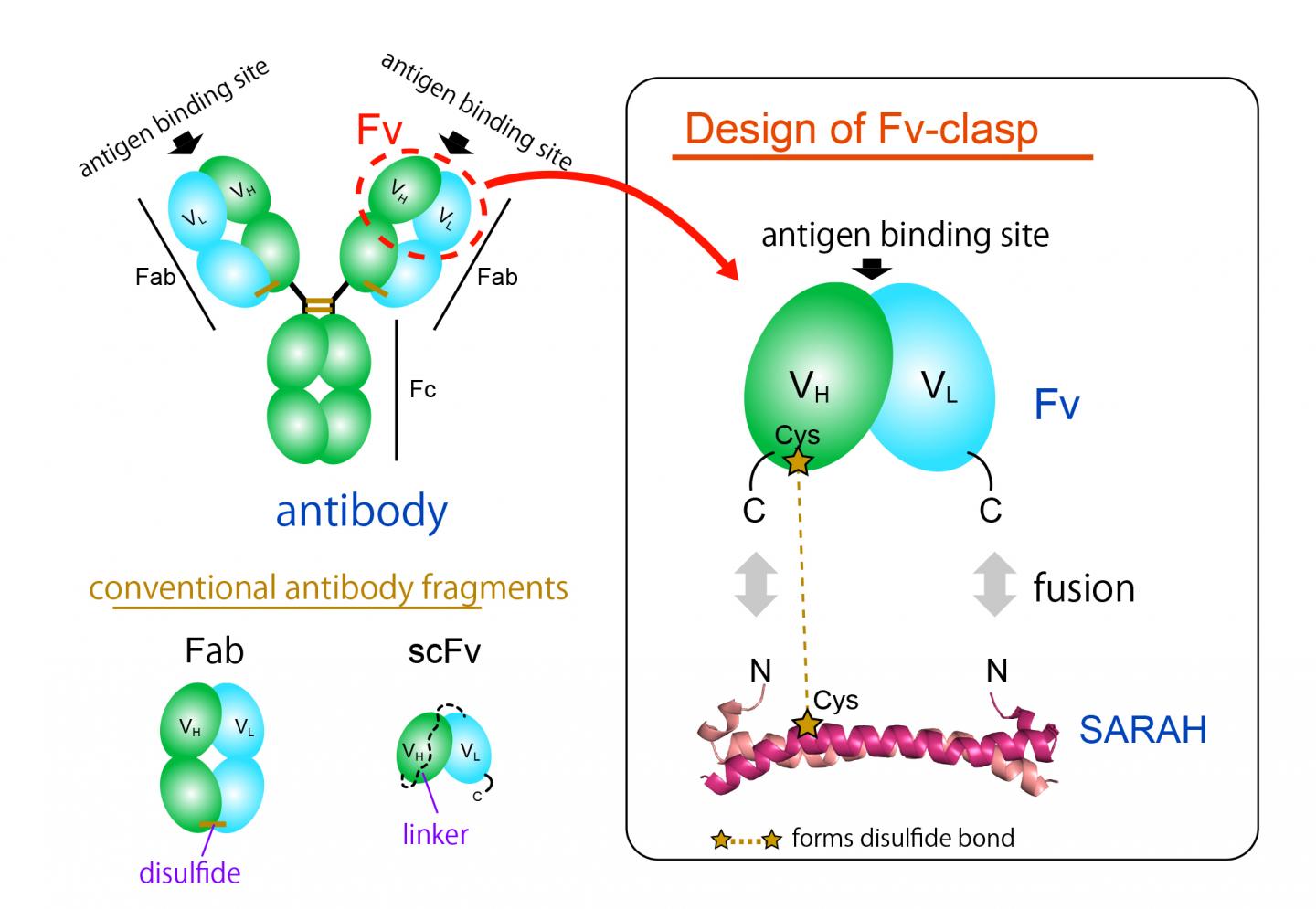
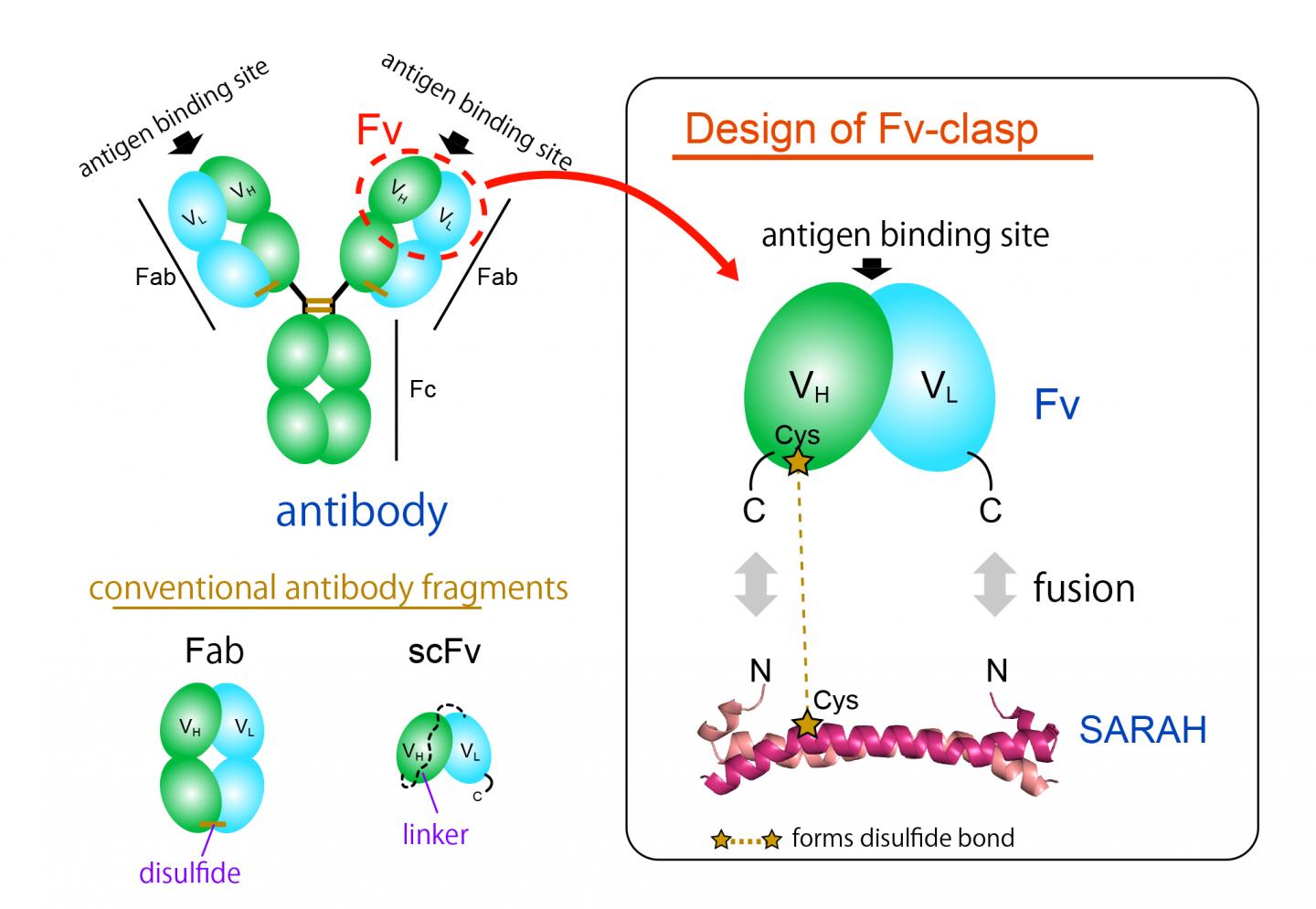
The most commonly used antibody fragment format is the Fab format, but it is difficult to produce in bacterial expression systems because of its large and complex structure. The Fv fragment of the antibody, which contains only one heavy and one light chain, would be ideal for this application thanks to its simple and small architecture. However, Fv is rarely used because the two chains dissociate easily, leading to a loss of function.
Now, a team of Osaka University researchers has designed a novel single-chain Fv fragment with improved production compatibility, stability and crystallizability, while maintaining the binding ability of the original molecule. They recently published their findings in Structure.
"We successfully produced a new fragment by fusing an anti-parallel coiled-coil structure derived from a particular domain of a human enzyme, Mst1 kinase, to the antigen-binding sites of an antibody," study lead author Takao Arimori explains. "The resulting chimeric molecule, Fv-clasp, was functionally and structurally equivalent to the Fv of the original antibody."
Notably, switching from Fab to Fv-clasp format markedly enhanced the antibody-assisted crystallization of two biologically important proteins, the extracellular domains integrin α6β1 and sorLA. Integrin α6β1 plays a significant role in the attachment of iPS cells and ES cells as well as many cancer cells to the basement membrane, an extracellular protein network foundation present in tissues, while sorLA is protein receptor implicated in Alzheimer's disease.
"The universal applicability of the Fv-clasp design to large-scale and inexpensive production makes it desirable for industrial applications. Furthermore, its high heat stability is a great advantage for immunotherapies," corresponding author Junichi Takagi says. "Aside from the field of structural biology, we anticipate that the Fv-clasp design will contribute to the expansion of already eminent antibody use in both basic and applied sciences."
###
Media Contact
Saori Obayashi
[email protected]
81-661-055-886
@osaka_univ_e
http://www.osaka-u.ac.jp/en
Original Source
http://resou.osaka-u.ac.jp/en/research/2017/20170915_1 http://dx.doi.org/10.1016/j.str.2017.08.011