Alternative splicing flips enzyme core to enable anaerobic metabolism in low oxygen conditions
Credit: Margot Lautens
Unique molecular mechanics allowing parasites to thrive in the guts of one billion people open the door to new treatments that are safe for the host.
Around one billion people on the planet are infected with parasitic helminths, round worms that live in soil and colonize human guts through dirty water. The helminths owe their ability to survive in the low oxygen environment of the human gut to a unique enzyme variant, Donnelly Centre researchers have found.
The findings raise hopes of new treatments to quell growing resistance of parasites to available medications. Infections are common in less developed countries where they can leave long-lasting consequences on child development.
“When parasites are outside the body, which they are for a part of their lifecycle, they breathe oxygen just like we do,” says Andrew Fraser, a senior author and a professor of molecular genetics in the Donnelly Centre for Cellular and Biomolecular Research at U of T’s Faculty of Medicine. “We were trying to understand how these parasites survive inside the human gut where there’s almost no available oxygen.”
The study was also co-led by Gustavo Salinas, a professor at Universidad de la República in Uruguay, and Jennifer Shepherd, a professor at Gonzaga University in the U.S.
The findings have been published in e-Life, an online journal for life-sciences.
Most animals, including humans, make energy through aerobic, or oxygen-dependent metabolism, with the help of a molecule called ubiquinone, or UQ. When they are inside their host, parasitic helminths switch to an unusual type of anaerobic metabolism that burns a related molecule called rhodoquinone, or RQ.
In their previous study, Fraser’s team uncovered that UQ and RQ are made from different precursor molecules by the same enzyme called COQ2. But how does COQ2 know to use the UQ precursor when there’s oxygen around but use the RQ precursor when there’s no oxygen?
“Somehow there has to be a switch,” says Fraser. “If we could understand how that switch works and if we could take a small compound and interfere with that switch, prevent it from making RQ, that might be a way to kill a parasite in humans.”
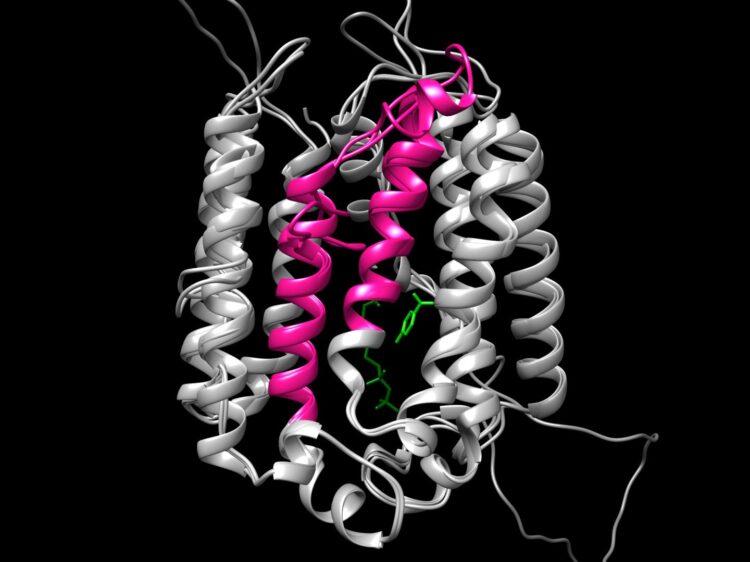
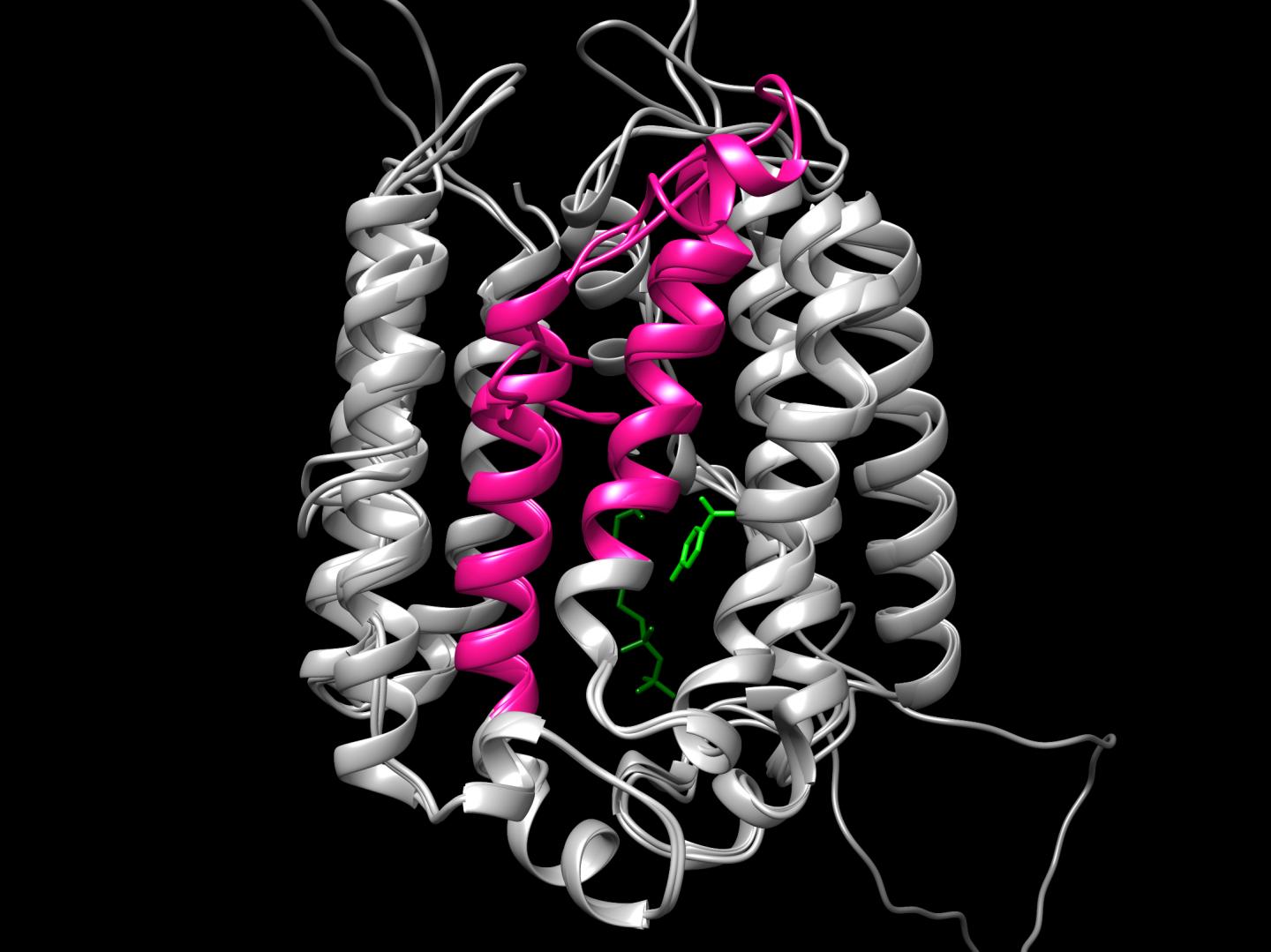
First clues emerged when Michael Schertzberg, a research technician in the lab, noticed that helminths produce two protein variants of COQ2. The variants are made by alternative splicing, a process through which gene coding segments, or exons, are variably included into templates for protein synthesis, allowing for diverse proteins to be encoded by the same gene. The two COQ2 variants are identical but for a small part encoded by two mutually exclusive exons, dubbed A and E. These are exactly the same size — flipping from the A variant to the E variant is like switching a block in a complicated Lego structure.
The researchers next engineered C. elegans worm strains producing either enzyme variant alone to test their ability to make UQ and RQ. Although not a parasite, C. elegans is a highly related helminth that also uses rhodoquinone. They found that the worms lacking the E variant lost their ability to make RQ and could no longer survive without oxygen.
Genome scanning across diverse animal lineages further strengthened the idea that the E variant is required for life without oxygen. The E variant is not even encoded in the COQ2 gene of most animals, including humans, who need air to live. It is only found in helminths and a few other species known to make RQ, such as oysters and other marine organisms, where it is likely an adaptation to changing oxygen levels in tidal environments.
Importantly, when they looked at the parasitic helminths Ascaris and Strongyloides stercoralis, they found that they also make and switch to the E variant when they are inside the host.
June Tan, a lead co-author and an expert in alternative splicing, has rarely seen in helminths two alternatively spliced variants with such distinct functions, like flipping a switch.
“For me the most surprising finding was how restricted the E variant was to just those species that make RQ,” says Tan, who is a postdoctoral fellow the lab.
“We think alternative splicing switches the enzyme core around the catalytic site so that it allows them to use a different precursor molecule to make RQ versus UQ.”
When Margot Lautens, a PhD student in the lab, computationally laid each variant over the reference molecular structure of the enzyme, she indeed found that the A and E exons encode a core segment which is crucial for the catalytic activity. The researchers think that when oxygen levels dip, the enzyme flips its inner core from the prevalent A form to the less common E form which can make RQ and sustain a parasite’s life.
The finding opens a therapeutic opportunity to specifically target the enzyme in the parasite without touching its counterpart in the host.
“If you look at the A form of COQ2, it looks the same in every animal. An inhibitor would act on human too,” says Fraser.
“But the E variant has key differences and you could target just that form. This gives us a beautiful way to help us find inhibitors that will hit specifically the E form and that’s what we’re doing now.”
The research was supported by funding from the Canadian Institutes for Health Research and Agencia Nacional para la Innovación y la Investigación ANII in Uruguay.
Follow us on LinkedIn and Twitter to keep up with Donnelly Centre news.
Media Contact
Jovana Drinjakovic
[email protected]
Original Source
https:/