Credit: University of Bristol
- Halo Therapeutics Ltd is preparing for clinical trials into pivotal, cost-effective antiviral treatments for COVID-19, after discovering a molecule which changes the shape of the virus’s spike protein and in so doing inhibits the virus’ ability to enter cells.
- Studies show the treatments are potentially ‘pan-corona antivirals’ in that they will work against all coronavirus strains – including the highly contagious ‘UK (Kent)’, ‘South African’ and ‘Brazilian’ variants.
- The company is preparing for clinical trials. If approved, the antivirals could be used by patients globally at the first sign of COVID-19 symptoms – stopping the virus in its tracks.
A team of top scientists from the University of Bristol have announced the formation of a new biotech company that is developing ground-breaking and newly patented potential treatments for coronavirus.
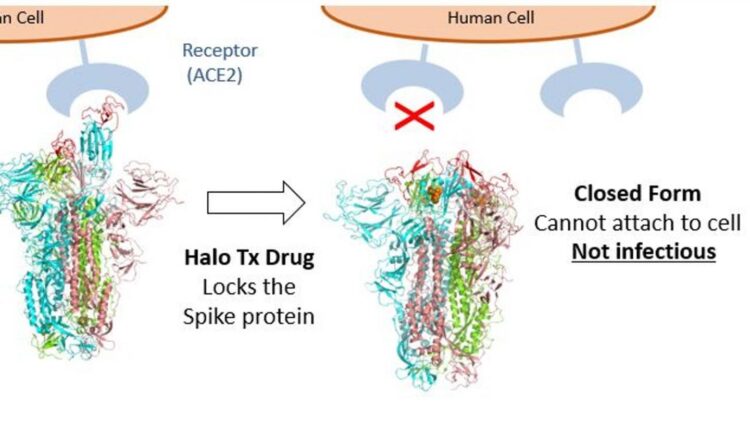
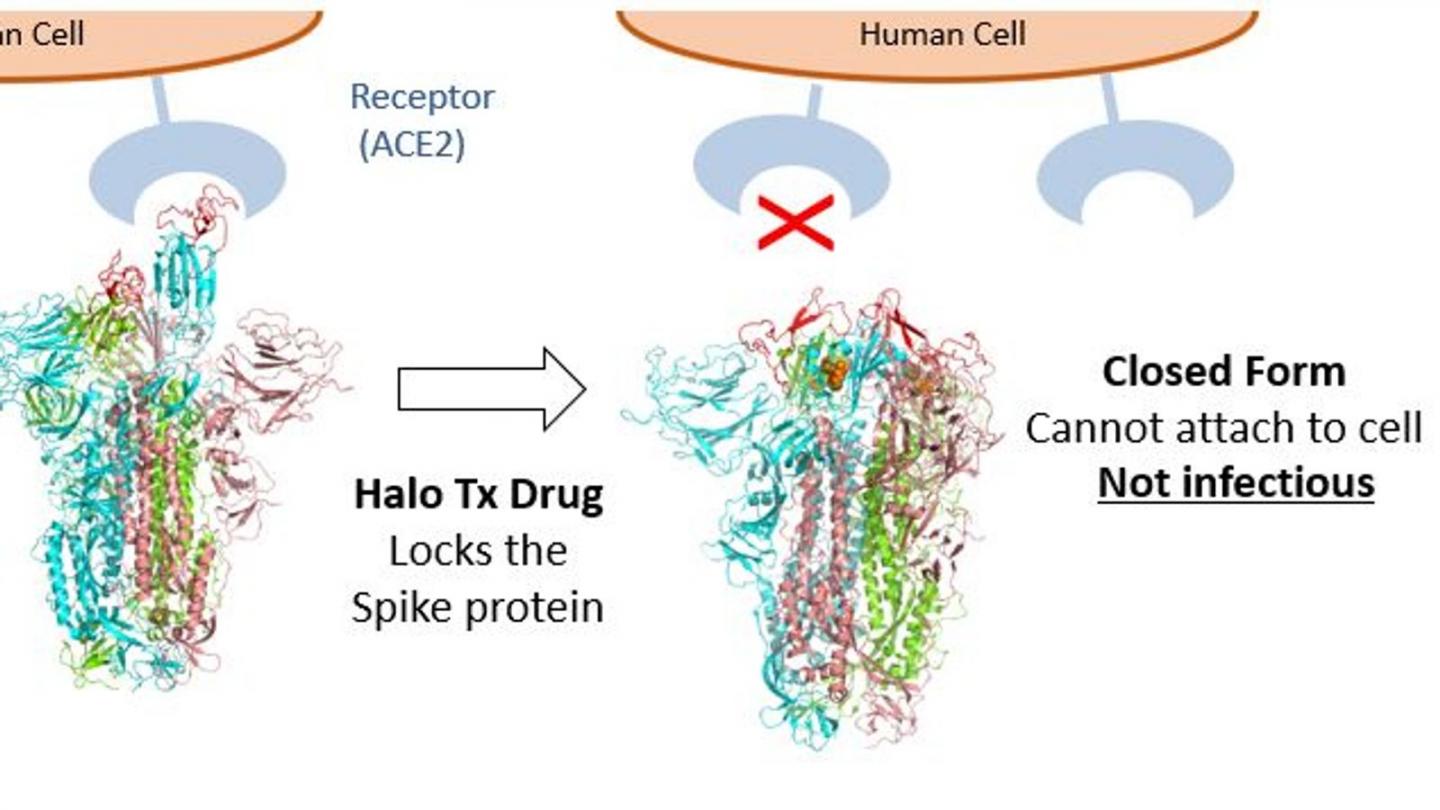
Bristol-based Halo Therapeutics is founded by the team of leading scientists who made a recent breakthrough discovery, which was published in Science. They found that exposing the SARS-CoV-2 (coronavirus) virus to a free fatty acid called linoleic acid locks the virus’s spike protein into a closed, non-infective form stopping it in its tracks.
The company is now preparing to make an application to start clinical trials with infected patients*. If proven to be effective, the antivirals could be used by people of all ages worldwide at the first sign of COVID-19 symptoms, or if they have been in contact with someone with the virus, preventing the virus from taking hold and stopping further transmission.
Lab studies indicate the antiviral will work against all pathogenic coronavirus strains including the highly contagious ‘UK (Kent)’, ‘South African’ and ‘Brazilian’ variants by preventing the virus from penetrating cells in the nose, throat and lungs. The treatments under development by Halo Therapeutics include a nasal spray and an asthma-type inhaler, and offer the possibility of a game-changing pan-coronavirus antiviral to treat patients at all stages of the disease and to reduce the transmission of the virus.
The Halo Therapeutics team is currently engaging investors to help finance multiple parallel clinical trials. If approved, the antiviral treatments could potentially start rolling out to patients globally.
Professor Imre Berger, Director of the Max Planck-Bristol Centre for Minimal Biology at Bristol and one of the team leading the drug’s development, explained: “The aim of our treatment is to significantly reduce the amount of virus that enters the body and to stop it from multiplying. Then, even if people are infected with the virus or exposed to it, they will not become ill because the antiviral prevents the virus from spreading to the lungs and beyond. Importantly, because the viral load will be so low it will likely also stop transmission.”
Professor Christiane Berger-Schaffitzel from Bristol’s School of Biochemistry, added: “Our vision is that at the first sign of the disease, whether you come into contact with someone who has COVID-19 or you have early symptoms, you would self-medicate at home to stop the virus in its tracks and prevent you from getting ill.”
Professor Adam Finn from Bristol Medical School and Bristol Vaccine Centre said: “As the virus mutates there is a real risk that presently available vaccines diminish in their protective effect and people could develop the disease again. We need an array of readily-available, cost-effective antiviral treatments that work across all virus strains and complement vaccination efforts.”
###
Media Contact
Joanne Fryer
[email protected]