Researchers block two cancer cell signaling pathways and slow tumor growth
Credit: Case Western Reserve School of Medicine
Blocking two molecular pathways that send signals inside cancer cells could stave off esophageal adenocarcinoma (EAC), the most common esophageal malignancy in the United States, according to new research out of Case Western Reserve University School of Medicine. Researchers identified the pathways using advanced computational and genetic analyses of tumor biopsies from EAC patients. They found 80 percent of tumors had unusually active genes related to two specific pathways, and that exposing the cells to pathway inhibitors stymied EAC tumor growth in mice.
Results published in Gastroenterology point to two signaling pathways (controlled by JNK and TGF-beta proteins, respectively) as contributing to EAC tumors. The pathways represent molecular chain reactions that were overactive in patient tumor cells, but not in biopsies from patients with non-cancerous esophageal conditions, including Barrett’s Esophagus. Harmful effects of these pathways could be reduced by turning down JNK or TGF-beta activity. “These findings suggest a rationale for testing JNK/TGF-beta-targeted therapies as a new treatment approach in this increasingly prevalent and lethal cancer,” said senior author Kishore Guda, DVM, PhD, associate professor in the Case Comprehensive Cancer Center.
Only 20 percent of patients diagnosed with EAC survive five years, according to recent National Cancer Institute estimates. Patients struggle to swallow as tumors and cancer cells narrow their esophagus. Some require nasogastric feeding tubes in end stages of disease. Limited available treatments to shrink tumors include surgery, radiation, or chemotherapy, but the majority of EAC tumors are resistant.
“Targeted therapies are virtually non-existent,” Guda said. “Treatment advancements are also slowed because we don’t know exactly what molecular signals drive EAC pathogenesis.”
In the new study, Guda and colleagues collected 397 biopsy specimens to find common mechanisms that underlie EAC tumor progression. They integrated computational and genetic analyses to identify signaling pathways highly active in EAC. They compared EAC biopsies to those collected from patients with conditions that often precede EAC, but who did not develop the cancer.
After finding JNK and TGF-beta pathways to be overactive only in EAC biopsies, they then incubated EAC tumor cells with therapeutic small molecules designed to block the pathways. Exposure to JNK or TGF-beta inhibitors reduced the ability of EAC cells to proliferate, migrate, or form tumors when transplanted into mice. Several mice had near total regression of tumor growth following treatment. Combining JNK and TGF-beta pathway inhibitor treatments further prevented cancer cell growth, but more studies are needed to understand synergy between the pathways during EAC progression.
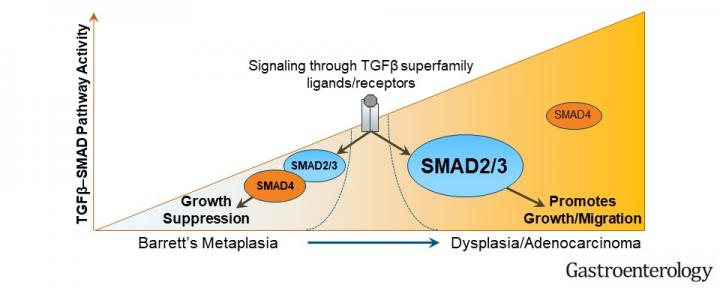
EAC tumor cells’ reliance on the TGF-beta pathway was unexpected given its widely recognized role as a cancer suppressor, said Guda’s co-senior author on the study, Vinay Varadan, PhD, assistant professor in the Case Comprehensive Cancer Center. The difference, Varadan said, potentially lies in different roles for TGF-beta in different stages of EAC development.
“In normal esophageal cells, TGF-beta acts as a gatekeeper by inhibiting uncontrolled cell growth,” Varadan said. “As EAC develops, TGF-beta switches from a growth suppressor to a growth promoter. This is unlike its function in other cancers such as those arising in the colon.” Varadan added, “Our unique application of advanced mathematical modelling that we developed allowed us to tease out these intricate mechanisms, which would have otherwise been missed.”
The results open a new targeted therapeutic avenue for EAC, and lay the foundation for studies in humans. Said Guda, “We are in the process of initiating a human clinical trial using an oral TGF-beta pathway inhibitor in EAC patients. Ultimately, we’ll use findings from that trial to guide development of TGF-beta inhibitors as a potential new targeted treatment option for EAC patients.”
###
The study included researchers from University Hospitals Cleveland Medical Center, Johns Hopkins School of Medicine, Washington University School of Medicine St. Louis, Vanderbilt Ingram Cancer Center, and the University of North Carolina Chapel Hill. Lead authors on the new study were Andrew Blum, MD, PhD, Srividya Venkitachalam, PhD, Durgadevi Ravillah, PhD, and Aruna Chelluboyina, PhD, all of Case Western Reserve University School of Medicine.
Blum A., et al. “Systems biology analyses reveal hyperactivation of transforming growth factor beta and JNK signaling pathways in esophageal cancer.” Gastroenterology. Published online February 12, 2019.
For more information about Case Western Reserve University School of Medicine, please visit: case.edu/medicine.
Media Contact
Ansley Gogol
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




