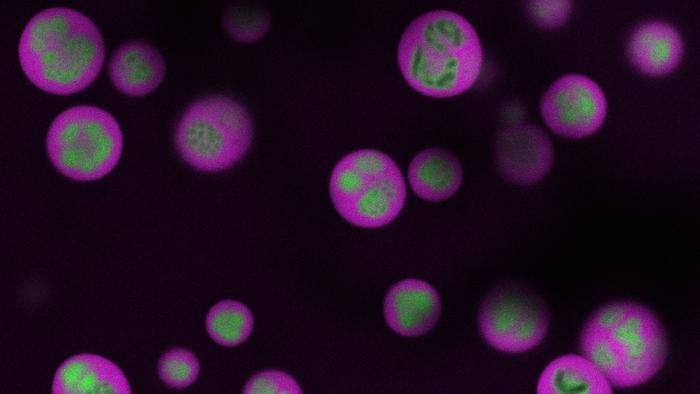
A groundbreaking technological advance from Princeton University has unveiled a previously inaccessible realm within the cell nucleus known as the nucleolus, a vital organelle responsible for the earliest stages of protein synthesis. This cellular “factory” has long evaded direct observation due to its delicate and complex architecture, but the team led by Princeton engineers has now developed an innovative method that offers an unprecedented window into these inner workings. By combining cutting-edge imaging and genomics tools, they have succeeded in mapping the intricate spatial and temporal choreography of RNA molecules within the multilayered nucleolus without compromising the cell’s structural integrity.
Proteins, the molecular workhorses of life, are synthesized through a finely tuned process beginning with the assembly of ribosomes inside the nucleolus. Ribosomes, complex macromolecular machines, translate RNA blueprints into functional proteins, orchestrating nearly every cellular activity. Despite decades of intensive research, the nucleolus’s dynamic, multiphase nature has obscured our understanding of how ribosomal components organize and mature within its compartments. Traditional biochemical approaches fractured the cell and obliterated this fragile milieu, effectively blinding scientists to the stepwise assembly occurring within.
The innovation from the Brangwynne laboratory sidesteps these limitations by analyzing live-cell RNA dynamics. The investigators track RNA trafficking and transformation through the nucleolus’s stratified architecture, which is comprised of distinct liquid-like phases reminiscent of oil droplets suspended in water. Each internal layer plays a defined role in ribosome production: RNA emerges in the innermost region, progressively assembling into ribosomal subunits, then migrates outward through the middle and outer layers before exiting the nucleolus to participate in protein synthesis. This technology captures snapshots of these processes in situ, illuminating the kinetic and structural nuances that guide ribosome maturation.
.adsslot_LTkYh3MwXo{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_LTkYh3MwXo{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_LTkYh3MwXo{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
In a landmark achievement, the team not only mapped native nucleolar architecture but engineered an artificial nucleolus—a simplified biomimetic model that replicates the functional hallmarks of its natural counterpart. This engineered system provides a powerful testbed for dissecting how various factors influence nucleolar assembly and function, allowing for controlled perturbation experiments and mechanistic insights. From this platform, researchers anticipate uncovering fundamental design principles governing intracellular phase separation and macromolecular complex formation.
Delving into the nucleolus’s multiphase organization revealed surprising behaviors linked to its biophysical properties. The discrete layers exhibit variable surface tensions and differential material compositions, creating a compartmentalized environment where ribosomal RNA (rRNA) processing and subunit assembly occur with exquisite spatial precision. Disruptions to RNA processing unbalance these forces, inducing profound structural rearrangements manifesting as inverted or fragmented nucleoli. Such observations strongly implicate RNA maturation as a central architect shaping nucleolar morphology by tuning interfacial tensions and phase organization.
By selectively interfering with RNA processing steps, the team observed the nucleolus either develop abnormal “necklace-like” formations or even invert its layered structure, indicating a delicate interplay between biochemical reactions and physical forces. These structural transformations underscore that nucleolar integrity depends not only on molecular makeup but also on dynamic material properties driven by ongoing RNA metabolism. This insight enhances our understanding of how membraneless organelles maintain order and adaptability within the crowded intracellular space.
Collaborations with leading ribosome biologists expanded the exploration into the functional consequences of nucleolar perturbations. Using DNA plasmids to induce the formation of designer nucleoli in living cells, the researchers validated that these exogenous structures closely mimic natural ribosome assembly kinetics and phase behavior. They replicated inside-out nucleolar arrangements analogous to those seen experimentally during defective RNA processing, reinforcing the concept that nucleolar order is tightly coupled to RNA quality control.
Indeed, the implications of this technology extend into disease realms. Cancer cells dramatically increase ribosome synthesis, yet the detailed orchestration of nucleolar processes in malignancy remains obscure. With this precise spatial-temporal map of nucleolar dynamics, researchers can now probe how oncogenic shifts influence the nucleolus’s internal landscape. Pinpointing stages sensitive to disruption may unveil novel therapeutic targets that selectively impair tumor growth by destabilizing aberrant ribosome production.
The strength of this work lies not only in its technical sophistication but also in its multidisciplinary approach encompassing chemical and biological engineering, molecular biology, and biophysics. The collaborative framework, supported by extensive institutional and funding partnerships, exemplifies how integrative science accelerates fundamental discoveries with far-reaching impact. By elucidating the molecular blueprint of nucleolar organization, the study paves the way for innovations in synthetic biology, drug discovery, and our broader understanding of cellular organization.
This pioneering research, published in the prestigious journal Nature, represents a monumental stride in cellular biology. By illuminating the previously “invisible” processes that blueprint the protein-making machinery, Princeton’s team has opened new avenues for interrogation and intervention. The nucleolus, once a “blobby” enigma within the nucleus, now reveals itself as a dynamic, phase-separated factory with precisely regulated workflows. These insights herald a transformative era where intracellular condensates can be mapped, modeled, and manipulated with exquisite detail, promising breakthroughs in health and disease alike.
Subject of Research: Cells
Article Title: Mapping and engineering RNA-driven architecture of the multiphase nucleolus
News Publication Date: 2-Jul-2025
Web References: http://dx.doi.org/10.1038/s41586-025-09207-4
References: Brangwynne C.P., Quinodoz S., Jiang L. et al. (2025) Nature
Image Credits: Holly Cheng/Brangwynne Lab
Keywords: nucleolus, ribosome assembly, RNA processing, biomolecular condensates, phase separation, cellular compartmentalization, synthetic nucleolus, ribosomal RNA, nucleolar structure, membrane-less organelles
Tags: advanced genomics toolscellular organelle researchcellular structural integrity preservationdynamic cellular processeslive-cell RNA dynamicsmultidisciplinary research approachnucleolus imaging technologyPrinceton University scientific innovationprotein factory observationprotein synthesis monitoringribosome assembly processRNA molecule mapping techniques