The human brain keeps changing throughout a person’s lifetime. New connections are continually created while synapses that are no longer in use degenerate. To date, little is known about the mechanisms behind these processes. Jülich neuroinformatician Dr. Markus Butz has now been able to ascribe the formation of new neural networks in the visual cortex to a simple homeostatic rule that is also the basis of many other self-regulating processes in nature. With this explanation, he and his colleague Dr. Arjen van Ooyen from Amsterdam also provide a new theory on the plasticity of the brain — and a novel approach to understanding learning processes and treating brain injuries and diseases.
The brains of adult humans are by no means hard wired. Scientists have repeatedly established this fact over the last few years using different imaging techniques. This so-called neuroplasticity not only plays a key role in learning processes, it also enables the brain to recover from injuries and compensate for the loss of functions. Researchers only recently found out that even in the adult brain, not only do existing synapses adapt to new circumstances, but new connections are constantly formed and reorganized. However, it was not yet known how these natural rearrangement processes are controlled in the brain. In the open-access journal PLOS Computational Biology, Butz and van Ooyen now present a simple rule that explains how these new networks of neurons are formed.
“It’s very likely that the structural plasticity of the brain is the basis for long-term memory formation,” says Markus Butz, who has been working at the recently established Simulation Laboratory Neuroscience at the Jülich Supercomputing Centre for the past few months. “And it’s not just about learning. Following the amputation of extremities, brain injury, the onset of neurodegenerative diseases, and strokes, huge numbers of new synapses are formed in order to adapt the brain to the lasting changes in the patterns of incoming stimuli.”
Activity regulates synapse formation
These results show that the formation of new synapses is driven by the tendency of neurons to maintain a ‘pre-set’ electrical activity level. If the average electric activity falls below a certain threshold, the neurons begin to actively build new contact points. These are the basis for new synapses that deliver additional input — the neuron firing rate increases. This also works the other way round: as soon as the activity level exceeds an upper limit, the number of synaptic connections is reduced to prevent any overexcitation — the neuron firing rate falls. Similar forms of homeostasis frequently occur in nature, for example in the regulation of body temperature and blood sugar levels.
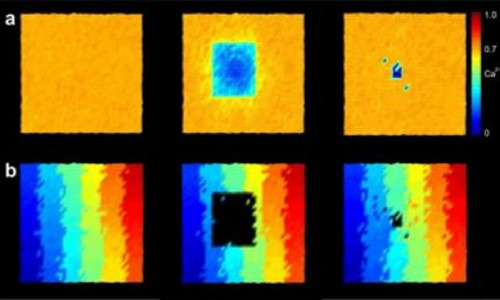
However, Markus Butz stresses that this does not work without a certain minimal excitation of the neurons: “A neuron that no longer receives any stimuli loses even more synapses and will die off after some time. We must take this restriction into account if we want the results of our simulations to agree with observations.” Using the visual cortex as an example, the neuroscientists have studied the principles according to which neurons form new connections and abandon existing synapses. In this region of the brain, about 10 % of the synapses are continuously regenerated. When the retina is damaged, this percentage increases even further. Using computer simulations, the authors succeeded in reconstructing the reorganization of the neurons in a way that conforms to experimental results from the visual cortex of mice and monkeys with damaged retinas.
The visual cortex is particularly suitable for demonstrating the new growth rule, because it has a property referred to as retinotopy: This means that points projected beside each other onto the retina are also arranged beside each other when they are projected onto the visual cortex, just like on a map. If areas of the retina are damaged, the cells onto which the associated images are projected receive different inputs. “In our simulations, you can see that areas which no longer receive any input from the retina start to build crosslinks, which allow them to receive more signals from their neighbouring cells,” says Markus Butz. These crosslinks are formed slowly from the edge of the damaged area towards the centre, in a process resembling the healing of a wound, until the original activity level is more or less restored.
Synaptic and structural plasticity
“The new growth rule provides structural plasticity with a principle that is almost as simple as that of synaptic plasticity,” says co-author Arjen van Ooyen, who has been working on models for the development of neural networks for decades. As early as 1949, psychology professor Donald Olding Hebb discovered that connections between neurons that are frequently activated will become stronger. Those that exchange little information will become weaker. Today, many scientists believe that this Hebbian principle plays a central role in learning and memory processes. While synaptic plasticity in involved primarily in short-term processes that take from a few milliseconds to several hours, structural plasticity extends over longer time scales, from several days to months.
Structural plasticity therefore plays a particularly important part during the (early) rehabilitation phase of patients affected by neurological diseases, which also lasts for weeks and months. The vision driving the project is that valuable ideas for the treatment of stroke patients could result from accurate predictions of synapse formation. If doctors knew how the brain structure of a patient will change and reorganize during treatment, they could determine the ideal times for phases of stimulation and rest, thus improving treatment efficiency.
New approach for numerous applications
“It was previously assumed that structural plasticity also follows the principle of Hebbian plasticity. The findings suggest that structural plasticity is governed by the homeostatic principle instead, which was not taken into consideration before,” says Prof. Abigail Morrison, head of the Simulation Laboratory Neuroscience at Jülich. Her team is already integrating the new rule into the freely accessible simulation software NEST, which is used by numerous scientists worldwide.
These findings are also of relevance for the Human Brain Project. Neuroscientists, medical scientists, computer scientists, physicists, and mathematicians in Europe are working hand in hand to simulate the entire human brain on high-performance computers of the next generation in order to better understand how it functions. “Due to the complex synaptic circuitry in the human brain, it’s not plausible that its fault tolerance and flexibility are achieved based on static connection rules. Models are therefore required for a self-organization process,” says Prof. Markus Diesmann from Jülich’s Institute of Neuroscience and Medicine, who is involved in the project. He heads Computational and Systems Neuroscience (INM-6), a subinstitute working at the interface between neuroscientific research and simulation technology.
Story Source:
The above story is based on materials provided by Forschungszentrum Juelich.