The hormone GIP regulates body weight and food intake via a receptor in the brain
Credit: Helmholtz Zentrum München
The GIP receptor in the central nervous system plays a crucial role in the regulation of body weight and food intake. This is shown by a recent study by Helmholtz Zentrum München, ETH Zurich and the German Center for Diabetes Research (DZD). The study, which has now been published in ‘Cell Metabolism‘, identifies new targets for the development of a drug treatment for obesity and type 2 diabetes.
Dual-agonists targeting the receptors for Glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are promising novel drug candidates for the treatment of obesity and diabetes. The new study shows how GIP decreases body weight. GIP is a hormone produced by the digestive tract. After food intake, GIP stimulates the release of insulin and thus lowers blood glucose levels. The hormone also has an effect on appetite regulation. However, the mechanisms and organs through which GIP affects body weight are unknown. Until now, it was unclear whether the GIP receptor should be activated or inhibited for body weight reduction and what role the brain plays in the effect of GIP. “The aim of our studies was to find out whether the GIP receptor in the brain plays a special role in the action of GIP,” said first author Qian Zhang of the Institute for Diabetes and Obesity at Helmholtz Zentrum München.
GIP lowers body weight through brain-mediated inhibition of food intake
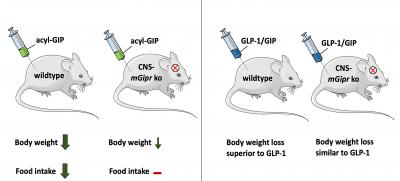
The researchers were able to show that the administration of GIP reduces body weight and food intake in wild-type mice, but not in mice that lack the GIP receptor in the central nervous system.
Does the hormone act on specific areas in the brain? To answer this question, the researchers investigated the brain activity of mice with diet-induced obesity after they had been treated with GIP. “This revealed increased neuronal activity in areas of the hypothalamus associated with appetite control,” said Professor Christian Wolfrum of ETH Zurich. The authors conclude that the central regulation of food intake via GIP also includes the activation of important neurons in the hypothalamus.
New targets for the development of a drug treatment for obesity and type 2 diabetes
The new findings are also important for the development of drug treatment for obesity and type-2 diabetes. Researchers at Helmholtz Zentrum München, together with Indiana University, have developed a new therapeutic approach for type-2 diabetes. They combined hormones in a single molecule that act equally at the receptors of the insulin-stimulating hormones GLP-1 and GIP. The dual agonist lowers body weight and improves blood glucose levels1. GLP-1/GIP dual-agonists are already in phase 3 clinical trials. Clinical studies showed that GLP-1/GIP reduces body weight to a greater extent than treatment with GLP-1 alone.
However, these marked differences in weight loss were not observed in mice lacking the GIP receptor in the CNS, says Dr. Timo Müller, last author of the new study and acting Director of the Institute for Diabetes and Obesity. Here, the GLP-1/GIP dual-agonist and the administration of GLP-1 equally reduce body weight. “Our research shows for the first time that the GLP-1/GIP dual-agonist requires the GIP receptor in the brain to reduce body weight and food intake,” said DZD-Researcher Dr. Müller. “These findings may aid in the development of novel drug targets that improve the signaling and effect of the GIP receptor. This could help to further increase the metabolic benefits of treatment with GIP and GLP-1/GIP.”
###
Scientific Contact:
Dr. Timo Müller
Helmholtz Zentrum München
German Research Center for Environmental Health (GmbH)
Institute for Diabetes and Obesity
Ingolstädter Landstraße 1
D-85764 Neuherberg
Phone: +49 89 3187-43278
Original publication:
Qian Zhang et al.: The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metabolism. DOI:https:/
Media Contact
Birgit Niesing
[email protected]
Original Source
https:/
Related Journal Article
http://dx.