Credit: Wade Van Horn
A team of scientists from ASU’s School of Molecular Sciences and the Biodesign Institute have recently published a study in Nature Communications that helps clarify the contributions to an ion channel’s temperature – dependent activation. This in turn should aid in the development of new types of non-addictive pain therapies.
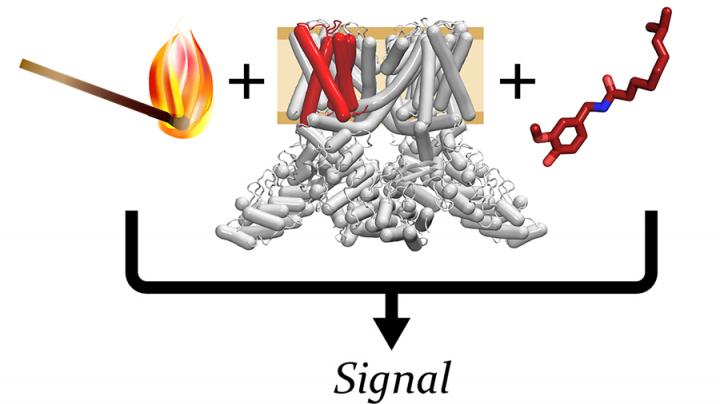
The ability to sense and respond to temperature is fundamental in biology. Ion channels are formed by membrane proteins that allow ions to pass through the otherwise impermeable lipid cell membrane, where they are used as a communication network.
“TRPV1 is an ion channel that is widely expressed in various tissues and plays a variety of roles in biology,” explains SMS professor Wade Van Horn, senior author of the current research. “It is best known for its role as the primary hot sensor in humans; it is the main way that we sense heat in our environment.”
Although important contributions have been made in the investigation of TRPV1 thermosensing, its mechanism has remained elusive.
TRPV1 is also a common taste and pain sensor, think spicy foods and pepper spray. Beyond these roles, it has been implicated in longevity, inflammation, obesity, and cancer. For decades it has been a target in the search for new types of pain medication, ones that are not addictive.
“However, to date, a common feature is that while TRPV1 targeting compounds can relieve pain, they also cause off-target effects, especially causing changes in body temperature, which has limited their utility. These off-target effects happen because TRPV1 is activated by many distinct stimuli, including ligands (i.e., capsaicin – the main ingredient in pepper spray), heat, and protons (acidic pH),” says Van Horn.
Also particularly limiting, is the uncertainty about the mechanisms that underlie temperature-sensing and how the different activation mechanisms are linked together.
This study used a variety of techniques, from cellular to atomic in nature, to investigate the domain of TRPV1 that is key to its ligand activation.
The techniques included Nuclear Magnetic Resonance spectroscopy experiments (like an MRI) aided by Brian Cherry (Associate Research Professional in the Magnetic Resonance Research Center), intrinsic fluorescence carried out in SMS associate professor Marcia Levitus’ lab. Levitus is also part of the Biodesign Center for Single Molecule Biophysics. Other techniques included far ultraviolet circular dichroism and temperature dependent electrophysiology.
Van Horn explains that this work identifies for the first time, both functionally and thermodynamically, that a particular region (of TRPV1) is crucial to heat activation. The team proposes, and provides experimental validation for, the heat activation mechanism and details a number of structural changes that happen as the temperature is changed.
This study provides a framework that the team anticipates will be foundational for future studies to further refine how we sense high temperatures and, importantly, how we can distinguish and target specific activation mechanisms that should promote the development of new types of non-addictive pain therapies.
All the interdisciplinary studies were completed at ASU. The team also included: Minjoo Kim, Nicholas Sisco and Jacob Hilton who are currently postdoctoral researchers at Columbia University, Barrow Neurological Institute and NIH respectively. Camila Montano and Wade Van Horn, are also part of the Biodesign Institute Virginia G. Piper Center for Personalized Diagnostics and Manuel (Mac) Castro is currently a doctoral student at Vanderbilt University.
###
Media Contact
Jenny Green
[email protected]




