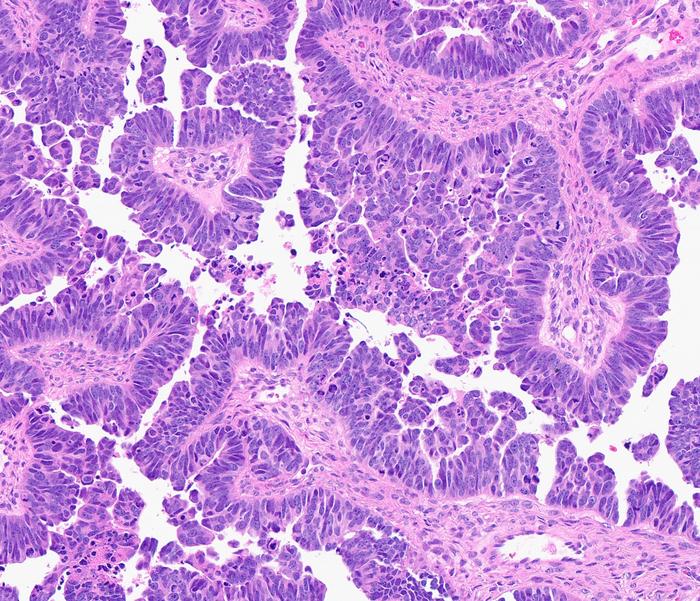
In a groundbreaking advancement in cancer biology, researchers at the University of Michigan Rogel Cancer Center have uncovered pivotal insights into the genetic mechanisms driving high-grade serous carcinoma (HGSC), a notoriously aggressive and lethal form of ovarian cancer. This investigative endeavor, recently published in the prestigious Proceedings of the National Academy of Sciences, elucidates the critical tumor-suppressive role of the gene CDK12 and explores innovative therapeutic strategies that could transform treatment paradigms for this deadly disease.
High-grade serous carcinoma stands as the predominant ovarian cancer subtype, often originating in the epithelium of the fallopian tubes before rapidly disseminating to the ovaries and other pelvic organs. Clinically, it presents immense challenges due to its advanced stage at diagnosis and a dismal prognosis, frequently demonstrating resistance to frontline chemotherapy regimens. The malignant complexity of HGSC is underscored by a heterogeneous genetic landscape marked by extensive genomic instability and multiple aberrations, among which alterations in CDK12 have now gained considerable attention.
The crux of the study centers on genetically engineered murine models replicating human HGSC features. The research team has innovatively expanded upon prior models by introducing quadruple gene inactivation, explicitly incorporating CDK12 deletions alongside three other known tumor suppressors in the mouse oviduct — an anatomical correlate to the human fallopian tube. This model has been instrumental in delineating the functional consequences of CDK12 loss, which remarkably accelerates tumor progression and exacerbates disease lethality, providing compelling evidence of CDK12’s tumor suppressor function in this context.
.adsslot_gsVtXFPWIi{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_gsVtXFPWIi{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_gsVtXFPWIi{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Notably, the inactivation of CDK12 did not merely intensify tumor proliferation; it simultaneously elicited a distinctive immune microenvironmental response. Researchers observed an increased infiltration of immune T cells within the tumor milieu, suggesting that CDK12 loss triggers immune activation pathways which might be therapeutically exploitable. This observation pivots the understanding of CDK12’s role beyond intrinsic cancer cell regulation, extending to its influence over tumor-immune dynamics.
Building upon these findings, the team identified a partner gene, CDK13, synergistic with CDK12, as a promising molecular target. Utilizing a specialized degrader compound capable of selectively degrading both CDK12 and CDK13 proteins, the researchers demonstrated significant tumor suppression in the murine models. This targeted approach, combined with immune checkpoint blockade therapies, yielded a pronounced reduction in tumor burden, heralding a potential combinatorial regimen that harnesses both genetic vulnerability and immune modulation in combating HGSC.
This research carries profound clinical implications. Current treatment of high-grade serous carcinoma heavily relies on cytotoxic chemotherapy, which, despite initial efficacy, often succumbs to tumor resistance mechanisms. The discovery that CDK12/13 degraders can not only suppress aggressive tumor growth but also potentiate immune responses offers a dual therapeutic angle that could transcend conventional chemotherapeutic strategies and address the substantial unmet need for effective interventions in chemotherapy-resistant patients.
Moreover, the study bridges gaps between disparate cancer types by revealing that CDK12 mutations are not exclusive to ovarian malignancies. Previous work from the same investigative group has implicated CDK12 as a driver in aggressive metastatic prostate cancer, where it accounts for approximately 7% of cases. In HGSC, CDK12 mutations occur in roughly 3% of tumors. This cross-cancer relevance amplifies the translational potential of CDK12/13-targeted therapies, suggesting broader applicability across oncology.
Delving into the molecular biology, CDK12 is a cyclin-dependent kinase intricately involved in the regulation of DNA damage response genes and the maintenance of genomic stability. Its functional impairment destabilizes transcriptional fidelity, precipitating genomic instability—a hallmark of cancer progression. The engineered mouse model vividly recapitulates these human pathobiological attributes, validating it as a robust platform for preclinical evaluation of novel therapeutic agents targeting this pathway.
The immune repercussion of CDK12 loss observed in this study is particularly noteworthy given the burgeoning field of immuno-oncology. Tumors with an enhanced immune infiltrate often respond more favorably to immunotherapies, an insight that could pave the way for integrating CDK12/13 inhibition with immune checkpoint inhibitors in clinical protocols. The interplay between genetic aberration-induced tumor aggression and concurrent immune activation opens avenues to exploit synthetic lethality and immune modulation synergistically.
Despite the promise, these findings remain at the preclinical stage. The CDK12/13 degrader employed in this study is yet to enter clinical trial phases. Continuous developmental efforts aim to optimize such molecules for human application, with the goal of initiating clinical evaluations that will ascertain safety, efficacy, dosing, and patient stratification criteria. The translational trajectory outlined by the team underscores the criticality of robust animal models in bridging laboratory discoveries and clinical reality.
This work also emphasizes the meticulous process of validating animal models to ensure faithful representation of human disease. Beyond histological features, researchers assess tumor development kinetics, genetic alterations, gene expression patterns, and tumor-immune microenvironment composition to authenticate model fidelity. Such comprehensive characterization ensures the reliability of therapeutic outcomes derived from these preclinical systems.
The philanthropic and governmental support underpinning this research includes notable grants from the National Cancer Institute, the U.S. Department of Defense, and the Prostate Cancer Foundation, signifying the high-impact nature and cross-institutional collaboration inherent in this endeavor. Additionally, intellectual property protections regarding CDK12/13 degraders signal active industry partnerships aimed at expediting drug development pipelines.
As the scientific community continues to grapple with the complexities of ovarian cancer, this study offers a beacon of progress — highlighting how unraveling the genetic circuitry of tumors not only deepens biological understanding but also catalyzes novel, targeted therapeutic opportunities. The integration of genetic insights with immune biology represents a frontier in precision oncology, one that holds promise for extending survival and improving quality of life for patients afflicted by high-grade serous carcinoma.
Subject of Research: Animals
Article Title: Defining CDK12 as a Tumor Suppressor and Therapeutic Target in Mouse Models of High-Grade Serous Carcinoma
News Publication Date: 9-Jun-2025
Web References:
University of Michigan Rogel Cancer Center
Michigan Medicine Cancer AnswerLine
References:
“Defining CDK12 as a Tumor Suppressor and Therapeutic Target in Mouse Models of High-Grade Serous Carcinoma,” PNAS. DOI: 10.1073/pnas.2426909122
Image Credits: Kathleen Cho, M.D.
Keywords: Ovarian cancer, Cancer genetics, Cancer research, Cancer
Tags: aggressive ovarian cancer researchcancer biology advancementsCDK12 gene role in cancerchemotherapy resistance in ovarian cancerfallopian tube origin of ovarian cancergenomic instability in HGSChigh-grade serous carcinoma insightsinnovative cancer therapeuticsmurine models of ovarian cancerovarian cancer geneticsovarian cancer treatment strategiestumor-suppressive mechanisms