LA JOLLA, CA—Our many different organ systems are in constant communication with each other. During exercise, for example, muscles send out signals to fat and liver tissue to release their energy sources. While these communication networks play a critical role in our bodies every day, it has been historically difficult to uncover such pathways. Scientists at Scripps Research, University of Southern California and elsewhere have now successfully created a model to label and track the protein signals that enable organ-to-organ communication.
Credit: Scripps Research and University of Southern California
LA JOLLA, CA—Our many different organ systems are in constant communication with each other. During exercise, for example, muscles send out signals to fat and liver tissue to release their energy sources. While these communication networks play a critical role in our bodies every day, it has been historically difficult to uncover such pathways. Scientists at Scripps Research, University of Southern California and elsewhere have now successfully created a model to label and track the protein signals that enable organ-to-organ communication.
As the researchers described in Open Biology on August 10, 2022, their new mouse model marks the proteins that a cell secretes and tracks their movement throughout the body. This novel technology could shape our molecular understanding of healthy versus diseased tissue, as well as the role that inter-organ communication plays in disease onset and progression.
“This new model can be likened to establishing a passport system in the body, as we are identifying where proteins are coming from and where they’re going,” says study co-first author Ilia Droujinine, PhD, Scripps fellow and principial investigator in the Department of Molecular Medicine at Scripps Research. “We can finally bring these interconnected communications networks to light, and then develop treatments based on this new knowledge.”
Researchers have used other methods, such as viral approaches, to understand protein secretion and the ways that organs communicate with each other. While these techniques have provided invaluable insight into the proteins expressed in an organism, they aren’t sensitive enough to label low-abundance proteins, or the origin and ultimate destination of protein interactions. But with this new model, scientists are now able to understand the exact path a particular protein takes.
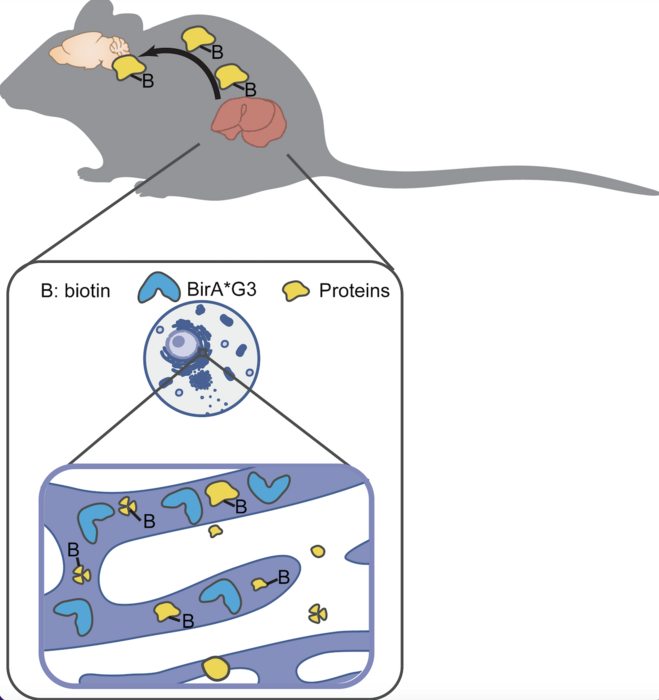
In the study, the researchers used an enzyme called BirA*G3, which labels secreted proteins with a biotin tag. These biotin labels were then detected in live mice using a method called quantitative mass spectrometry proteomics, which is used to measure proteins in a sample. This revealed where the proteins originated from and where they traveled to in the body.
When BirA*G3 was broadly activated throughout the body, the researchers found that all secreted proteins were successfully labeled, even low-abundance proteins with hormone-like properties. Likewise, when BirA*G3 was activated solely in the liver, only secreted proteins related to that organ system were highlighted— displaying the high specificity of the model as well.
“Given the central role of key secreted proteins such as insulin, there is a great deal of interest in identifying novel secreted proteins,” said Andrew McMahon, PhD, senior author of the study and chair of the Department of Stem Cell Biology and Regenerative Medicine at the University of Southern California. “Genome studies are suggesting that many new proteins remain to be characterized. We’re looking forward to a deep dive into this area now that we have validated the technology.”
There are countless research applications for this technology, Droujinine notes. With this type of model, scientists can start to map unexplored disease pathways and ultimately develop targeted treatments, as many diseases originate in a single organ and then eventually spread to others. Cancer, with its metastatic properties, is one example. For another, studies have shown that many of the health complications that arise from obesity could be due to faulty organ communication, yet many of the molecular mechanisms remain unknown.
“Any protein we discover that plays a role in disease has the potential be translated into a therapeutic,” adds Droujinine.
In addition to Droujinine and McMahon, authors of the study, “A genetic model for in vivo proximity labelling of the mammalian secretome,” include Rui Yang, Amanda S. Meyer, Jinjin Guo, Jill A. McMahon of the University of Southern California (USC); Namrata D. Udeshi, Dominique K. Carey, Charles Xu and Steven A. Carr of Broad Institute of Harvard and MIT; Yanhui Hu, David Rocco and Norbert Perrimon of Harvard Medical School; Qiao Fang of University of Toronto; Jihui Sha and James Wohlschlege of UCLA; Shishang Qin of Peking University; and Alice Y. Ting of Chan Zuckerberg Biohub.
This work was supported by a NIH Transformative R01 grant 5R01DK121409 to A.P.M., A.Y.T., S.A.C. and N.P., and HHMI funding to N.P. I.A.D. acknowledges support from the Ellen Browning Scripps Foundation.
About Scripps Research
Scripps Research is an independent, nonprofit biomedical institute ranked the most influential in the world for its impact on innovation by Nature Index. We are advancing human health through profound discoveries that address pressing medical concerns around the globe. Our drug discovery and development division, Calibr, works hand-in-hand with scientists across disciplines to bring new medicines to patients as quickly and efficiently as possible, while teams at Scripps Research Translational Institute harness genomics, digital medicine and cutting-edge informatics to understand individual health and render more effective healthcare. Scripps Research also trains the next generation of leading scientists at our Skaggs Graduate School, consistently named among the top 10 US programs for chemistry and biological sciences. Learn more at www.scripps.edu.
Journal
Open Biology
Article Title
A genetic model for in vivo proximity labelling of the mammalian secretome
Article Publication Date
10-Aug-2022




