RENTON, Wash. [January 25] – The demand for donated organs has already exceeded supply, with patients waiting months and sometimes years for a donor. With so many people testing positive for SARS-CoV-2, the virus that causes COVID-19, a new study published in the Transplant Infectious Diseases could provide help for the shortage of donated organs by using an organs from a SARS-CoV-2-positive donor.
Credit: Jason Goldman, M.D.
RENTON, Wash. [January 25] – The demand for donated organs has already exceeded supply, with patients waiting months and sometimes years for a donor. With so many people testing positive for SARS-CoV-2, the virus that causes COVID-19, a new study published in the Transplant Infectious Diseases could provide help for the shortage of donated organs by using an organs from a SARS-CoV-2-positive donor.
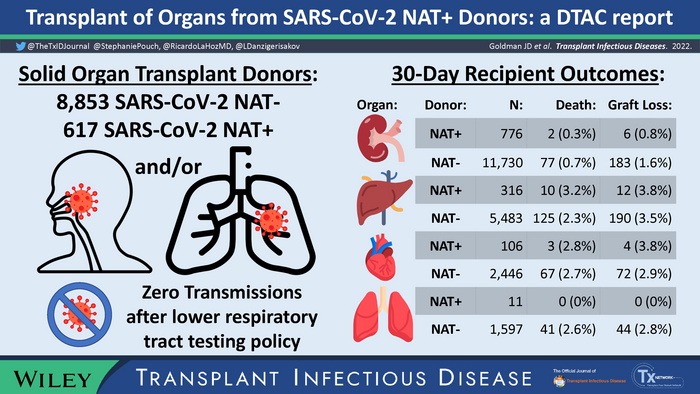
To evaluate the safety of transplants from SARS-CoV-2-positive organ donors, Providence Swedish researcher Jason Goldman, M.D., M.P.H. who is an infectious disease physician at the Organ Transplant and Liver Disease Center at Providence Swedish Hospital in Seattle, WA, led a group of more than two dozen national experts from the Organ Procurement and Transplantation Network (OPTN) ad hoc Disease Transmission Advisory Committee to conduct the largest study on the question of whether it was safe to receive an organ from a SARS-CoV-2 positive donor. The study involved an investigation of data including all U.S. donors and transplant recipients in the national database, as well as cases referred to the committee for review of possible transmission from donor to recipient. Their review assessed 30-day outcomes for 1,241 transplant recipients from a donor testing positive for SARS-CoV-2, and 21,948 organ donors without a positive virus test. The analysis found that non-lung transplant recipients from positive donors have 30-day graft and patient survival rates like those using donors negative for SARS-CoV-2.
The researchers also evaluated the impacts of a 2021 emergency policy requiring Organ Procurement Organizations to conduct lower respiratory track (LRT) testing for SARS-CoV-2. The policy was launched in late May 2021 after the Centers for Disease Control and Prevention (CDC) and the Disease Transmission Advisory Committee identified three cases where SARS-CoV-2 was passed to lung transplant recipients from the donor, after upper respiratory tract testing failed to identify the donors’ COVID-19 infections.
A retrospective analysis of all donors recovered and recipients transplanted in the U.S. looked at the national data between May 27, 2021, when the new LRT policy took effect, and January 31, 2022. Not only did the research team find that no probable or proven cases of COVID-19 transmission had occurred to recipients of non-lung organs (such as kidneys, livers and hearts), they also found zero transmissions of COVID-19 to lung transplant recipients after the new policy requiring lower respiratory track testing in lung donors took effect.
“Our findings are particularly heartening for patients and providers grappling with the dual challenges of an organ shortage and uncertainty about the safety of organ transplants during the COVID-19 pandemic,” explains Dr. Goldman. “Early guidance for transplant programs recommended avoiding donors who recently tested positive for SARS-CoV-2. However, this study provides key evidence that in most cases, transplants from SARS-CoV-2-positive donors can be used with excellent short-term outcomes.”
While the researchers point to the need for additional studies regarding long-term outcomes, this study provides much-needed evidence and reassurance for transplant recipients, donors, their loved ones and providers regarding transplant during the COVID-19 pandemic. .
About Providence Swedish
Providence Swedish has served the Puget Sound region since the first Providence hospital opened in Seattle in 1877 and the first Swedish hospital opened in 1910. The two organizations affiliated in 2012 and today comprise the largest health care delivery system in Western Washington, with 22,000 caregivers, eight hospitals and 244 clinics. A not-for-profit family of organizations, Providence Swedish provides more than $406 million in community benefit in the Puget Sound Region each year. The health system offers a comprehensive range of services and specialty and subspecialty care in a number of clinical areas, including cancer care, cardiovascular health, neurosciences, orthopedics, digestive health and women’s and children’s care
Journal
Transplant Infectious Disease
DOI
10.1111/tid.14013
Method of Research
Observational study
Subject of Research
People
Article Title
Transplant of organs from donors with positive SARS-CoV-2 nucleic acid testing: A report from the organ procurement and transplantation network ad hoc disease transmission advisory committee
Article Publication Date
24-Jan-2023
COI Statement
Jason D. Goldman reported contracted research from Gilead, Eli Lilly, and Regeneron, grants from Merck (BARDA), and collaborative services agreements with Adaptive Biotechnologies, Monogram Biosciences, and Labcorp; and served as a consultant, speaker, or advisory board member for Gilead, Eli Lilly, GSK, and Karius. Raymund R. Razonable received research grants (funds to the institution) from Gilead, Renegeron, and Roche; served as member of the DSMB of Novartis; served as consultant to Glaxo-Smith-Kline, Merck, Roche; and served as Board of Director of the American Society of Transplantation. Helen S. Te served as a consultant for CVS Caremark and Precision BioSciences. Anil J. Trindade served on the Lung National Scientific Advisory Board for CareDx, Inc. He has received funding support from CareDx, Inc. and Veloxis Pharmaceuticals. All other authors declare they have no disclosures or conflicts of interest.