Researchers from Heinrich Heine University Düsseldorf (HHU) and the University of Duisburg-Essen (UDE) have together succeeded in identifying and synthesising a group of molecules that can act against the cause of tuberculosis in a new way. In the scientific journal Cell Chemical Biology, they describe that the so-called callyaerins act against the infectious disease by employing a fundamentally different mechanism compared to antibiotic agents used to date.
Credit: HHU/Rainer Kalscheuer
Researchers from Heinrich Heine University Düsseldorf (HHU) and the University of Duisburg-Essen (UDE) have together succeeded in identifying and synthesising a group of molecules that can act against the cause of tuberculosis in a new way. In the scientific journal Cell Chemical Biology, they describe that the so-called callyaerins act against the infectious disease by employing a fundamentally different mechanism compared to antibiotic agents used to date.
The infectious disease tuberculosis is caused by the bacterium Mycobacterium tuberculosis (for short: M. tuberculosis). More than ten million people contract the disease worldwide every year. According to the World Health Organisation (WHO), 1.6 million people died of tuberculosis in 2021 alone. This makes it one of the most significant infectious diseases and, in particular in countries with inadequate healthcare systems, it represents a serious threat to the population.
Over time, M. tuberculosis has developed resistance to many antibiotics, making treatment increasingly difficult. There are currently only a few drugs available that are effective against resistant strains. Researchers are therefore seeking new antibacterial compounds and mechanisms of action as a basis for the development of completely new drugs.
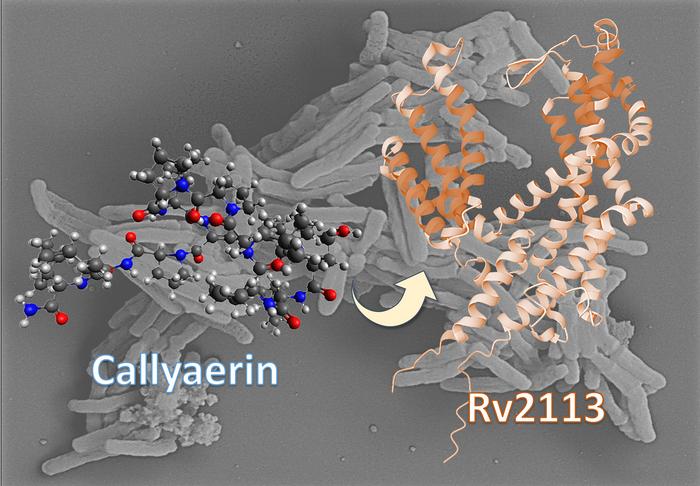
A research team headed by Professor Dr Rainer Kalscheuer from the Institute of Pharmaceutical Biology and Biotechnology at HHU and Professor Dr Markus Kaiser from the Center of Medical Biotechnology at UDE has identified one such fundamentally new approach involving callyaerins. Chemically, these natural substances of marine origin are classed as so-called cyclopeptides.
“We have succeeded in chemically synthesising the substance that occurs naturally in marine sponges in order to test its effect on tuberculosis bacteria in cell cultures. This has enabled us to produce new, more potent derivatives that do not exist in nature. Such chemical synthesis needs to be successful before a potential active agent can be used as a drug on a large scale,” explains Dr David Podlesainski from UDE, one of the two lead authors of the study that has now been published in Cell Chemical Biology.
The tuberculosis bacterium primarily infects human phagocytes, the so-called macrophages, in which the bacteria then multiply. The researchers have now discovered that callyaerins can inhibit the growth of the bacterium in human cells.
Emmanuel Tola Adeniyi, doctoral researcher at HHU and co-lead author of the study: “The callyaerins attack a specific membrane protein of M. tuberculosis called Rv2113, which is not essential for the viability of the bacterium. This comprehensively disrupts the metabolism of the bacterium, hindering its growth. By contrast, human cells remain unaffected by the callyaerins.”
Professor Kalscheuer, corresponding author of the study: “With the callyaerins, we have discovered a new mechanism of action. Unlike other antibiotics, these substances do not block vital metabolic pathways in the bacterial cell. Instead, they directly attack a non-essential membrane protein of the bacterium, which has not been considered as a potential target before.”
Professor Kaiser, the second corresponding author, focuses on a further perspective: “In further research work, we now need to clarify precisely how callyaerins interact with Rv2113 and how this interaction disrupts various cellular processes in such a way that M. tuberculosis can no longer grow. However, we have been able to show that non-essential proteins can also represent valuable target structures for the development of novel antibiotics.”
Original publication:
David Podlesainski, Emmanuel T. Adeniyi, Yvonne Gröner, Florian Schulz, Violetta Krisilia, Nidja Rehberg, Tim Richter, Daria Sehr, Huzhuyue Xie, Viktor E. Simons, Anna-Lene Kiffe-Delf, Farnusch Kaschani, Thomas R. Ioerger, Markus Kaiser and Rainer Kalscheuer. The anti-tubercular callyaerins target the Mycobacterium tuberculosis-specific non-essential membrane protein Rv2113. Cell Chemical Biology 31 (2024).
DOI: 10.1016/j.chembiol.2024.06.002
Journal
Cell Chemical Biology
DOI
10.1016/j.chembiol.2024.06.002
Article Title
The anti-tubercular callyaerins target the Mycobacterium tuberculosis-specific non-essential membrane protein Rv2113
Article Publication Date
8-Jul-2024




