A groundbreaking advance in molecular imaging promises to revolutionize how aggressive cancers are visualized and managed clinically. Researchers have developed a novel positron emission tomography (PET) tracer capable of rapidly detecting nectin-4, a protein frequently overexpressed in triple-negative breast cancer (TNBC) and urothelial bladder carcinoma (UBC). This innovative tracer enables same-day immuno-PET imaging, potentially transforming diagnostic protocols by delivering high-contrast, real-time images within hours of administration, as reported in the September 2025 issue of The Journal of Nuclear Medicine.
Triple-negative breast cancer remains a formidable challenge in oncology due to its lack of hormone receptors and HER2 expression, limiting targeted therapy options. Representing approximately 24% of newly diagnosed breast cancer cases, TNBC often exhibits aggressive behavior and poor prognosis. Similarly, urothelial bladder carcinoma accounts for roughly 90% of bladder cancers, frequently diagnosed at advanced stages, necessitating precise and early detection tools to improve patient outcomes. Both malignancies share common molecular markers, including the cell adhesion protein nectin-4, which has emerged as a promising therapeutic target.
Nectin-4 is implicated in a variety of tumorigenic processes, including cell proliferation and metastasis, rendering it a critical biomarker for aggressive cancers. However, the clinical utility of nectin-4-targeted therapies has been hindered by the lack of rapid, noninvasive techniques for patient stratification and treatment monitoring. Addressing this gap, the multidisciplinary team led by Weibo Cai, PhD, at the University of Wisconsin Madison and collaborators at Peking University First Hospital, engineered two PET tracers: one conjugated to a full-length antibody and another featuring a fragmented antibody format, both labeled with the radioisotope copper-64.
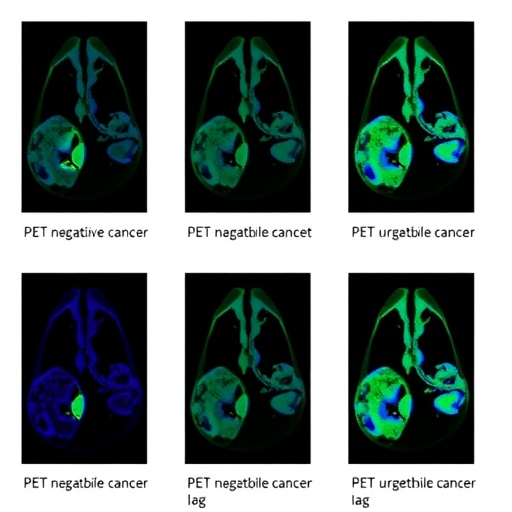
The study meticulously evaluated these tracers, [64Cu]Cu-NOTA-EV (full-length antibody) and [64Cu]Cu-NOTA-EV-F(ab′)2 (antibody fragment), through a comprehensive battery of assays. Initial in vitro experiments utilized flow cytometry and immunofluorescence to quantify nectin-4 expression across a panel of TNBC and UBC cell lines. Binding specificity and affinity were rigorously assessed via cellular uptake and competitive binding assays, confirming the tracers’ selective interaction with nectin-4-positive cells.
Subsequent in vivo investigations employed xenograft mouse models implanted with tumors exhibiting varying levels of nectin-4. Immuno-PET imaging revealed striking differences in kinetic profiles between the two tracers. Notably, the fragmented antibody tracer demonstrated rapid and targeted tumor accumulation, reaching peak signal intensity as early as four hours post-injection. This rapid uptake significantly enhanced tumor-to-background contrast, an essential parameter for accurate lesion delineation in clinical settings.
Pharmacokinetic analyses underscored the superiority of the [64Cu]Cu-NOTA-EV-F(ab′)2 tracer, which exhibited faster blood clearance and reduced nonspecific tissue retention relative to its full-length counterpart. This favorable profile not only facilitates dynamic imaging on the same day as tracer administration but also minimizes radiation dose to non-target organs—a crucial safety consideration in nuclear medicine.
Quantitative biodistribution studies further supported the tracer’s efficacy, demonstrating enhanced tumor-to-healthy tissue ratios over time. These metrics are vital for assessing the potential for precise tumor localization, therapeutic monitoring, and early response evaluation, thereby empowering clinicians to make informed treatment decisions swiftly and confidently.
The implications of this research extend beyond TNBC and UBC. The modular nature of the antibody fragment and the versatility of isotopic labeling pave the way for adaption to a wide range of oncological targets and molecular signatures. This approach heralds a new era in personalized medicine, wherein rapid and accurate visualization of tumor biomarkers informs targeted therapy, improving both efficacy and patient quality of life.
Expert commentary from Lei Kang, MD, PhD, highlights the transformative potential of these findings. The ability to perform same-day immuno-PET imaging represents a paradigm shift, enabling real-time, noninvasive interrogation of tumor biology. This capability could substantially expedite clinical workflows and reduce the logistical burden associated with traditional imaging methods that require extended waiting periods between tracer administration and scanning.
Crucially, this study aligns with broader efforts in molecular imaging to enhance specificity and speed without compromising safety. By leveraging the finely tuned properties of antibody fragments and advanced radiochemistry, the research charts a course toward faster, safer, and more patient-friendly imaging modalities, essential for managing aggressive cancers that demand prompt intervention.
The development of [64Cu]Cu-NOTA-EV-F(ab′)2 as a reliable PET tracer exemplifies the intersection of molecular biology, radiochemistry, and clinical oncology. Its capacity to provide high-resolution images of nectin-4 expression within hours offers a promising tool for patient stratification, real-time treatment monitoring, and potentially, early detection of recurrence or metastatic spread.
Future research directions may encompass the expansion of this imaging platform to incorporate novel radioisotopes and explore additional molecular targets across diverse cancer types. Moreover, integration with theranostic approaches could enable simultaneous diagnostic imaging and delivery of targeted therapeutics, ushering in a new frontier of precision oncology.
In summary, this innovative PET imaging technique represents a significant leap forward in the noninvasive visualization of aggressive cancers. It promises to reduce diagnostic latency, tailor treatment strategies more effectively, and ultimately improve clinical outcomes for patients afflicted with challenging malignancies like triple-negative breast cancer and urothelial bladder carcinoma.
Subject of Research: PET imaging of nectin-4 expression in triple-negative breast cancer and urothelial bladder carcinoma using novel radiolabeled antibody fragments.
Article Title: [64Cu]Cu-NOTA-EV-F(ab′)2 Enables Same-Day Immuno-PET Imaging of Nectin-4 in Triple-Negative Breast and Urothelial Bladder Cancers
News Publication Date: September 20, 2025
Web References:
The Journal of Nuclear Medicine: https://jnm.snmjournals.org/
DOI: http://dx.doi.org/10.2967/jnumed.125.270132
References:
Huang W., Li L., Chao F., Yang Q., Kang L., Mixdorf J.C., Engle J.W., Hsu J.C., Cai W. “[64Cu]Cu-NOTA-EV-F(ab′)2 Enables Same-Day Immuno-PET Imaging of Nectin-4 in Triple-Negative Breast and Urothelial Bladder Cancers,” Journal of Nuclear Medicine, 2025.
Image Credits: Wenpeng Huang, University of Wisconsin–Madison and Peking University First Hospital.
Keywords: Molecular imaging, PET, positron emission tomography, breast carcinoma, triple-negative breast cancer, urothelial bladder cancer, nectin-4, immuno-PET, antibody fragments, radiotracers, precision medicine.
Tags: advancements in nuclear medicineaggressive cancer visualization techniqueschallenges in oncology treatment optionsearly detection of bladder cancerinnovative PET tracer developmentmolecular imaging techniquesnectin-4 as a cancer biomarkerreal-time cancer imaging advancementssame-day PET imaging for cancerstargeted therapies for TNBCtriple-negative breast cancer diagnosticsurothelial bladder carcinoma imaging