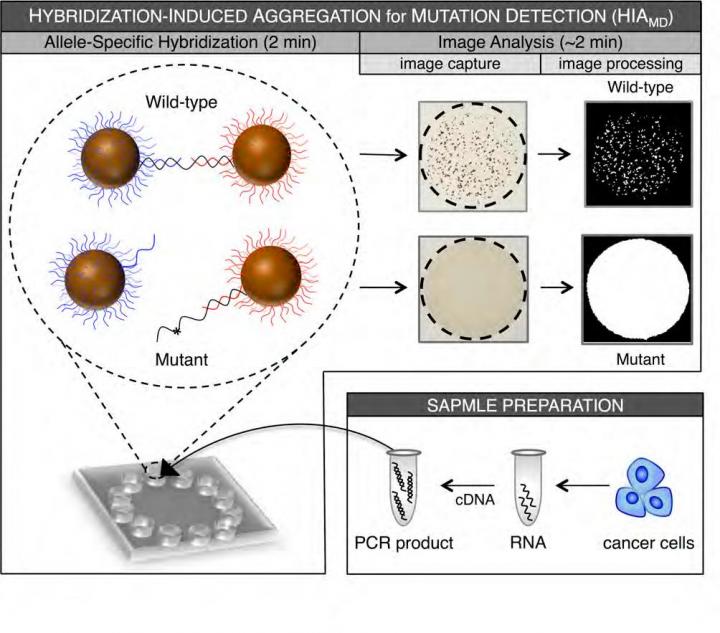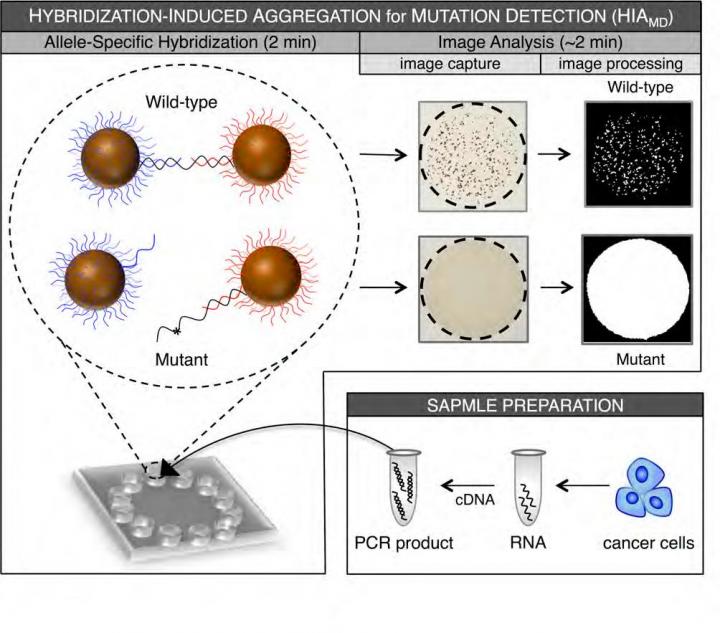
Philadelphia, PA, June 8, 2016 – A new technology suitable for practical clinical testing can detect KRAS gene mutations in lung and colorectal cancers and could thereby facilitate targeted therapies, according to a new report in The Journal of Molecular Diagnostics.
The identification and functional analysis of tumor-specific genetic alterations suggest opportunities to exploit genetic mutations as predictors of therapeutic response and guide a more effective patient treatment regime. KRAS mutations represent a powerful biomarker for predicting treatment sensitivity in patients with non-small-cell lung cancer and colorectal cancer. The protein product of the normal KRAS gene is involved primarily in regulating cell division.
“Targeted therapies are a growing trend in basic and clinical cancer research, and for good reason — the potential for improved treatment outcomes and cost savings is tremendous,” explained lead investigator Kimberly A. Kelly, PhD, of the Department of Biomedical Engineering, University of Virginia, Charlottesville, VA. “However, the effective implementation of a targeted therapeutic regime requires a practical means for preemptive molecular characterization of the cancer. In an effort to fulfill the unmet clinical demand for a methodology amenable to the requirements of routine testing, we developed a novel approach that provides simple, rapid, and inexpensive detection of point mutations.”
Conventional mutation testing technologies are not ideal for routine clinical screening of KRAS mutations because they often involve complex, time-consuming processes and/or costly instrumentation. Investigators have therefore developed and tested a new technique for lung and colorectal cancer samples that can be used in routine testing.
The new technique described in the study uses hybridization-induced aggregation (HIA) technology for mutation detection (HIAMD), which enables the detection of common KRAS mutations in less than 10 minutes following PCR amplification. HIA is a bead-based DNA-detection technology that is scalable for a microchip platform.
The investigators analyzed 20 lung and colorectal tumors and compared the results using this new technique with results from the more expensive and cumbersome sequencing method. The results of KRAS mutation screening using this technique were 100% in agreement with the results derived from sequencing. In addition, a sample with only 25% KRAS mutant content could be detected in a background of wild-type DNA, consistent with the detection limit reported using the sequencing method.
“These results indicate the validity of HIAMD as a mutation-testing technology suitable for practical clinical testing,” said Dr. Kelly. “Importantly, the analysis is performed in a manner that is both rapid and cost effective. The current direction of clinical oncology research suggests that a technology such as HIAMD will continue to be a highly relevant and valued analytical tool for the facilitation of individualized therapeutic strategies, and the successes here indicate the potential to apply this technology for the routine analysis of other important genetic markers.”
###
Media Contact
Eileen Leahy
[email protected]
732-238-3628
@elseviernews
https://www.elsevier.com/
The post New mutation-testing technology has potential to guide targeted lung and colorectal cancer therapies appeared first on Scienmag.