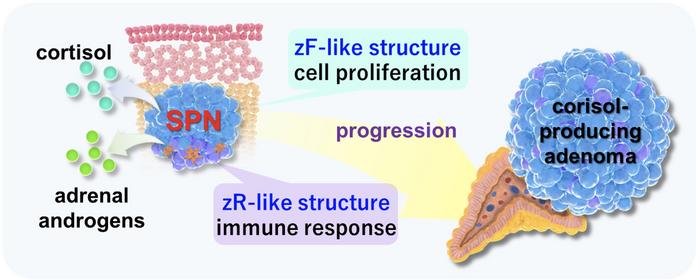
Fukuoka, Japan—Researchers from Kyushu University’s Faculty of Medical Sciences report on new insights into the mechanisms of how adrenal gland tumors are formed. The team identified a new type of tumor cell population that they termed ‘steroids-producing nodules’ or SPNs, that exhibits the unique characteristic of producing two different hormones. Specific structures in SPNs were found to lead to cortisol-producing adenomas, or CPAs, noncancerous tumors that produce excessive cortisol.
Credit: Kyushu University/Ogawa Lab
Fukuoka, Japan—Researchers from Kyushu University’s Faculty of Medical Sciences report on new insights into the mechanisms of how adrenal gland tumors are formed. The team identified a new type of tumor cell population that they termed ‘steroids-producing nodules’ or SPNs, that exhibits the unique characteristic of producing two different hormones. Specific structures in SPNs were found to lead to cortisol-producing adenomas, or CPAs, noncancerous tumors that produce excessive cortisol.
Their findings, published in eBioMedicine, provide clues into the formation and maintenance of the human adrenal cortex, which can lead to better treatments in diseases linked to its dysfunction.
Nobody likes the feeling of being under stress, your heart rate rises, your muscles tense, and your body starts twitching with energy. One of the key chemical compounds behind this is cortisol, known colloquially as the ‘stress hormone.’ Cortisol is a family of steroid hormones known as ‘adrenal steroid hormones’ and are produced within the small glands above the kidneys called the adrenal cortex.
The adrenal cortex is critical in producing the vital hormones regulating everything from your sleep-wake cycle, blood pressure, and even sexual development. Naturally, disruptions in the adrenal gland have been linked to a wide range of diseases.
“For example, tumors that can develop in the adrenal cortex can lead to the production of excessive amounts of certain hormones. One type of tumor we study are cortisol-producing adenomas, or CPAs, which are noncancerous tumors that produce excessive cortisol,” explains Research Fellow Tazuru Fukumoto, the first author of the study. “While researchers have found several genetic mutations linked to CPA, how exactly these tumors develop remained unclear.”
The human adrenal cortex is made of three layers, with each layer producing specific hormones: the zona glomerulosa that produce aldosterone, zona fasciculata (zF) that produce cortisol, and the zona reticularis (zR) that produce adrenal androgens.
The team began by collecting tissue samples from patients with adrenal tumors and conducted histopathologic tests along with the latest genetic analysis. They found structures that exhibited a unique two-layered zF and zR-like structure, producing both cortisol and adrenal androgens.
“We called these structures steroids-producing nodules, or SPNs. SPNs had mutations in the gene GNAS, that is known to cause CPAs. Further analyses showed that the zF-like quality enhanced cell proliferation, but the zR-like structure had a tumor suppressive effect. We also found that this zF-like structure was the main cause for the development of CPAs” continues Fukumoto. “In summary, when cells of the adrenal cortex acquire the GNAS gene mutation, they develop into SPNs, wherein their zF-like structure contributes to them becoming CPAs.”
The team hopes their findings can lead to better understanding and treatment of adrenocortical tumors and the diseases they are linked to.
“Steroid based drugs are vital in the treatment of many diseases including autoimmune diseases like rheumatoid arthritis, and allergic diseases like asthma. However, long-term use will atrophy of the adrenal cortex,” concludes Professor Yoshihiro Ogawa who led the study. “We hope our results will advance our understanding of the layered structure of the adrenal cortex and can lead to the prevention and treatment of adrenal cortex atrophy in the future.”
###
For more information about this research, see “Steroids-producing nodules: a two-layered adrenocortical nodular structure as a precursor lesion of cortisol-producing adenoma,” Tazuru Fukumoto, Hironobu Umakoshi, Norifusa Iwahashi, Tatsuki Ogasawara, Maki Yokomoto-Umakoshi, Hiroki Kaneko, Masamichi Fujita, Naohiro Uchida, Hiroshi Nakao, Namiko Kawamura, Yayoi Matsuda, Ryuichi Sakamoto, Takashi Miyazawa, Masahide Seki, Masatoshi Eto, Yoshinao Oda, Yutaka Suzuki, Seishi Ogawa, and Yoshihiro Ogawa eBioMedicine, https://doi.org/10.1016/j.ebiom.2024.105087
About Kyushu University
Kyushu University is one of Japan’s leading research-oriented institutes of higher education since its founding in 1911. Home to around 19,000 students and 8,000 faculty and staff, Kyushu U’s world-class research centers cover a wide range of study areas and research fields, from the humanities and arts to engineering and medical sciences. Its multiple campuses—including one of the largest in Japan—are located around Fukuoka City, a coastal metropolis on the southwestern Japanese island of Kyushu that is frequently ranked among the world’s most livable cities and historically known as Japan’s gateway to Asia. Through its Vision 2030, Kyushu U will ‘Drive Social Change with Integrative Knowledge.’ Its synergistic application of knowledge will encompass all of academia and solve issues in society while innovating new systems for a better future.
Journal
EBioMedicine
DOI
10.1016/j.ebiom.2024.105087
Method of Research
Experimental study
Subject of Research
Human tissue samples
Article Title
Steroids-producing nodules: a two-layered adrenocortical nodular structure as a precursor lesion of cortisol-producing adenoma
Article Publication Date
2-Apr-2024



