Chemical modifications of RNA molecules, such as m6A, can critically impact gene expression, influencing various aspects of cancer development and progression. However, while studies into m6A modification of messenger RNA (mRNA) have been extensive, exploration of its impact on lncRNAs, especially within the context of PDAC, has been relatively limited.
Credit: Genes & Diseases
Chemical modifications of RNA molecules, such as m6A, can critically impact gene expression, influencing various aspects of cancer development and progression. However, while studies into m6A modification of messenger RNA (mRNA) have been extensive, exploration of its impact on lncRNAs, especially within the context of PDAC, has been relatively limited.
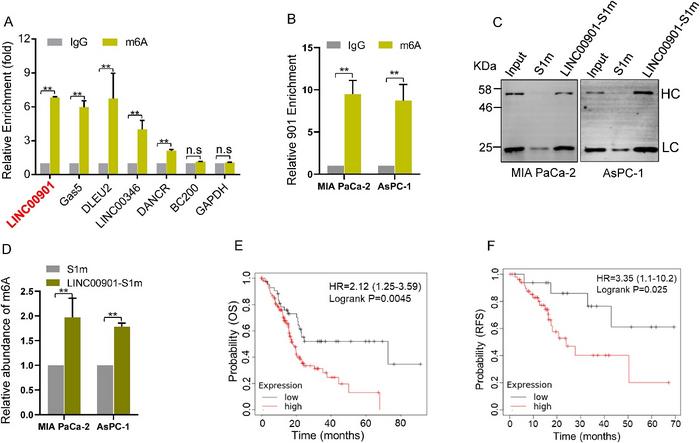
In an innovative study published in the Genes & Diseases journal, a team from the The Children’s Hospital, Zhejiang University School of Medicine, People’s Hospital of Hangzhou Medical College and University of Mississippi Medical Center employed a methylated RNA immunoprecipitation (MeRIP) strategy to uncover the role of LINC00901, an m6A-modified long noncoding RNA (lncRNA), in promoting the proliferation, survival, and invasiveness of pancreatic ductal adenocarcinoma (PDAC) cells, thus leading to tumor growth. Intriguingly, the study suggests that the m6A reader protein YTHDF1 negatively regulates LINC00901 expression. The team identified two m6A sites on LINC00901 essential for its interaction with YTHDF1. Their function was underscored when mutations at these sites reduced interaction, thereby emphasizing the significance of m6A modification in LINC00901’s oncogenic role. The study further unveils a critical LINC00901-IGF2BP2-MYC axis, driving PDAC progression in an m6A-dependent manner, thereby illuminating a potential new therapeutic target. The researchers suggest the m6A machinery as a promising therapeutic avenue, hinting at the potential for improved patient response to treatment through combining a checkpoint inhibitor with YTHDF1 deficiency. Moreover, with m6A modification implicated in the regulation of both innate and adaptive immune cells, the possibility of developing immunotherapies targeting this pathway emerges.
This study marks a significant advancement in understanding how RNA modifications, such as m6A, impact gene expression and contribute to cancer development. These findings offer fresh insights into the role of m6A modification in lncRNA in the context of PDAC, enhancing our understanding of the disease’s progression and opening up potential new pathways for treatment. By exploring this RNA modification, the research expands the horizon of possibilities for targeted cancer therapies.
###
References
Funding information
National Natural Science Foundation of China (82072703, 81772575, 81972455),
US Department of Defense (CA170314).
DOI
10.1016/j.gendis.2022.02.014
About Genes & Diseases
Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch.
Journal
Genes & Diseases
DOI
10.1016/j.gendis.2022.02.014
Subject of Research
Not applicable
Article Title
N6-methyladenosine modified LINC00901 promotes pancreatic cancer progression through IGF2BP2/MYC axis
Article Publication Date
11-May-2023



