In rodent studies, method reduced likelihood of further spinal cord trauma while delivering large doses of potentially reparative stem cells; the approach may have utility for multiple neurodegenerative conditions
Credit: UC San Diego Health Sciences
Writing in the journal Stem Cells Translational Medicine, an international research team, led by physician-scientists at University of California San Diego School of Medicine, describe a new method for delivering neural precursor cells (NSCs) to spinal cord injuries in rats, reducing the risk of further injury and boosting the propagation of potentially reparative cells.
The findings are published in the Jan. 29, 2020 print issue.
NSCs hold great potential for treating a variety of neurodegenerative diseases and injuries to the spinal cord. The stem cells possess the ability to differentiate into multiple types of neural cell, depending upon their environment. As a result, there is great interest and much effort to use these cells to repair spinal cord injuries and effectively restore related functions.
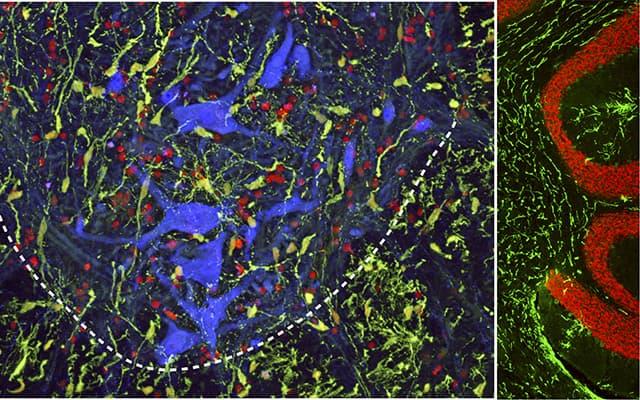
But current spinal cell delivery techniques, said Martin Marsala, MD, professor in the Department of Anesthesiology at UC San Diego School of Medicine, involve direct needle injection into the spinal parenchyma — the primary cord of nerve fibers running through the vertebral column. “As such, there is an inherent risk of (further) spinal tissue injury or intraparechymal bleeding,” said Marsala.
The new technique is less invasive, depositing injected cells into the spinal subpial space — a space between the pial membrane and the superficial layers of the spinal cord.
“This injection technique allows the delivery of high cell numbers from a single injection,” said Marsala. “Cells with proliferative properties, such as glial progenitors, then migrate into the spinal parenchyma and populate over time in multiple spinal segments as well as the brain stem. Injected cells acquire the functional properties consistent with surrounding host cells.”
Marsala, senior author Joseph Ciacci, MD, a neurosurgeon at UC San Diego Health, and colleagues suggest that subpially-injected cells are likely to accelerate and improve treatment potency in cell-replacement therapies for several spinal neurodegenerative disorders in which a broad repopulation by glial cells, such as oligodendrocytes or astrocytes, is desired.
“This may include spinal traumatic injury, amyotrophic lateral sclerosis and multiple sclerosis,” said Ciacci.
The researchers plan to test the cell delivery system in larger preclinical animal models of spinal traumatic injury that more closely mimic human anatomy and size. “The goal is to define the optimal cell dosing and timing of cell delivery after spinal injury, which is associated with the best treatment effect,” said Marsala.
###
Co-authors include: Kota Kamizato and Takahiro Tadokoro, UC San Diego and University of Ryukyus, Japan; Michael Navarro and Silvia Marsala, UC San Diego; Stefan Juhas, Jana Juhasova, Hana Studenovska and Vladimir Proks, Czech Academy of Sciences; Tom Hazel and Karl Johe, Neuralstem, Inc.; and Shawn Driscoll, Thomas Glenn and Samuel Pfaff, Salk Institute.
Disclosure: Martin Marsala is the scientific founder of Neurgain Technologies, Inc., which has licensed technologies from UC San Diego based on Marsala’s research. Marsala has equity interest in the company, and also serves as a consultant, for which he receives compensation.
Media Contact
Scott LaFee
[email protected]
858-249-0456
Related Journal Article
http://dx.




