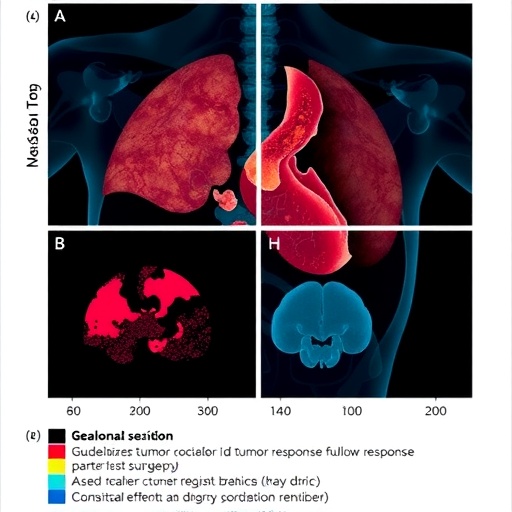
Researchers at the Johns Hopkins Kimmel Cancer Center and the Bloomberg~Kimmel Institute for Cancer Immunotherapy have announced a groundbreaking advancement in the evaluation of tumor responses to neoadjuvant therapy. This therapy, administered before surgery to shrink tumors, has been pivotal across numerous cancer types. However, the assessment of tumor response has faced significant variability due to disparate scoring systems used by pathologists worldwide. To address this critical challenge, the team has developed a unified, cross-cancer framework that standardizes how pathologists evaluate residual viable tumor, necrosis, and tumor regression following presurgical treatment.
This initiative, partly funded by the National Institutes of Health and The Mark Foundation, was carried out in collaboration with the Society for Immunotherapy of Cancer (SITC) and the International Neoadjuvant Melanoma Consortium (INMC). The updated guidelines, recently published in the prestigious journal Annals of Oncology, represent the first pan-tumor consensus designed to harmonize pathological assessment after neoadjuvant therapy. By refining and building upon immune-related criteria initially established for lung cancer in 2018 and expanded in 2020, the consortium ensures the guidelines incorporate half a decade of clinical application, feedback, and new reproducibility data.
The core innovation lies in the introduction of standardized parameters to assess residual viable tumor (RVT), necrosis, and regression—histological markers of treatment response that include inflammation and the signature traits of wound healing within tumor tissue. This comprehensive approach supersedes multiple tumor-specific systems, which previously complicated clinical interpretation and trial comparisons. With neoadjuvant therapies rapidly expanding across cancer types, including immunotherapy regimens targeting checkpoints such as PD-1, establishing a reliable, reproducible framework for pathological response is indispensable for clinical decision-making and research.
Julie Stein Deutsch, M.D., an assistant professor at Johns Hopkins and lead author of the study, underscores the clinical significance of this unified approach. She highlights that pathologic response is becoming a cornerstone in predicting long-term patient survival and an increasingly important endpoint in clinical trials. The disparity in scoring systems across tumor types has hindered the ability to draw consistent conclusions from diverse studies. The new guidelines offer a common language, greatly facilitating data consolidation, cross-trial comparison, and regulatory evaluation.
The genesis of these harmonized guidelines traces back to meticulous research analyzing approximately 500 tumor specimens treated with anti-PD-1 immunotherapies, either as monotherapy or combined with chemotherapy or targeted agents. Remarkably, the researchers observed analogous histopathological patterns of tumor regression irrespective of cancer origin. This uniformity signaled a pressing need to consolidate scoring criteria within a single, universally applicable framework to reduce confusion and improve reproducibility.
Janis Taube, M.D., senior author and director of dermatopathology at Johns Hopkins, elaborates on the practical advantages of the system. Given that most practicing pathologists are generalists rather than tumor-specific experts, navigating a patchwork of scoring guidelines is inefficient and prone to inconsistency. The newly proposed pan-tumor system not only streamlines workflow but also matches or outperforms existing tumor-specific methods in predicting patient survival outcomes, reinforcing its potential for broad adoption.
A hallmark feature of the updated guidelines is their demonstrated reproducibility across international, multi-institutional pathology teams. In a landmark study involving 14 pathologists, a brief standardized training enabled consistent scoring of residual viable tumor, necrosis, and regression across a range of solid tumor types and anatomical sites. This reproducibility extended to assessments of both surgical resection specimens and biopsy samples, confirming the system’s robustness and versatility. The findings were presented at the 2024 American Society of Clinical Oncology annual meeting, affirming the approach’s readiness for widespread clinical implementation.
The harmonized criteria bear a functional resemblance to the Response Evaluation Criteria in Solid Tumors (RECIST), a well-established method for radiographic tumor assessment used globally. This parallel allows clinicians and researchers to integrate pathological and imaging data coherently, thereby enhancing the precision of treatment response evaluations. The ability to standardize data collection is especially critical as neoadjuvant and perioperative therapies—those given before and after surgery—become standard across an expanding array of cancers.
Central to this initiative is the emphasis on reproducibility as a prerequisite for clinical utility. Dr. Deutsch emphasizes that treatment decisions and clinical trials depend on different pathologists arriving at comparable scores for identical specimens. To facilitate this, the research team has developed comprehensive training materials designed to ensure consistent application of the guidelines. The Society for Immunotherapy of Cancer is collaborating with the Johns Hopkins researchers to disseminate these resources widely, ensuring global access to standardized protocols.
The consortium anticipates that as long-term survival data from patients treated under this new scoring paradigm accumulate, further refinement of clinically relevant thresholds for residual viable tumor will become possible. These thresholds will be tailored for specific tumor types to enhance prognostic accuracy and guide therapeutic choices more precisely. This evolving framework thus represents a dynamic tool, adaptable to emerging clinical evidence and therapeutic advances.
This groundbreaking effort involves contributions from an extensive network of researchers, including experts in dermatology, pathology, oncology, immunotherapy, and bioinformatics. The team integrates expertise from institutes worldwide, supported by grants and funds from renowned organizations, including the Mark Foundation for Cancer Research, Bloomberg~Kimmel Institute, National Institutes of Health, and several international cancer research councils.
Importantly, the researchers maintain transparency regarding potential conflicts of interest, disclosing consultancy roles, research funding, and patents related to predictive methodologies in pathology. Such disclosures affirm the commitment to ethical standards, ensuring that the development and application of these guidelines are conducted with scientific integrity.
In summation, this unified, pan-tumor pathological evaluation framework marks a pivotal step toward improving the precision and consistency of neoadjuvant therapy response assessments. With validated reproducibility and broad applicability across multiple solid tumor types, the guidelines are set to revolutionize clinical cancer care and translational research. By harmonizing pathologic parameters akin to radiographic standards, the framework provides a robust foundation to accelerate oncologic innovation and optimize patient outcomes worldwide.
Subject of Research: Pathological assessment standardization of tumor response to neoadjuvant therapy across solid tumor types
Article Title: Updated Consensus Guidelines and Reproducibility Study for Pan-Tumor Pathologic Assessment of Neoadjuvant Therapy Response
News Publication Date: November 4, 2025
Web References: https://www.annalsofoncology.org/article/S0923-7534(25)04965-8/fulltext, https://www.annalsofoncology.org/article/S0923-7534(26)00038-4/fulltext
Keywords: Cancer risk, Cancer genetics, Neoadjuvant therapy, Residual viable tumor, Tumor regression, Pathologic response, Immunotherapy, PD-1 inhibitors, Pan-tumor assessment, Reproducibility, Clinical trial endpoints, Oncology
Tags: Annals of Oncology publicationcancer immunotherapy advancementscollaboration in cancer researchcross-cancer frameworkInternational Neoadjuvant Melanoma Consortiumneoadjuvant therapy guidelinespathological assessment consensusresidual viable tumor criteriastandardized cancer assessmenttumor regression evaluationtumor response measurement