Epigenetic Research Offers Hope for Colorectal Cancer Treatment
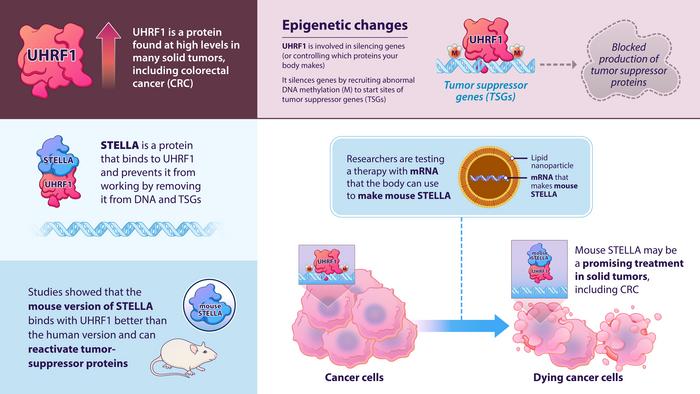
Recent findings from groundbreaking research conducted by experts from the Johns Hopkins Kimmel Cancer Center and the Chinese Academy of Sciences illuminate a promising avenue for treating colorectal cancer and potentially other solid tumors. Central to this research is a relatively obscure protein found in mice, known as STELLA, which appears to disrupt harmful epigenetic alterations to genes linked to the development of human colorectal cancer cells. This innovative approach harnesses the power of epigenetics to address cancer in a new and potentially transformative way.
The research, published in the peer-reviewed journal “Nature Communications,” details how the mouse form of STELLA has shown a superior ability to impair tumor growth compared to its human equivalent. This notable disparity prompted the research team to focus on understanding the specific amino acids responsible for the enhanced efficacy of the mouse protein. By leveraging these insights, they have undertaken a bold drug strategy aimed at treating colorectal cancer in both cell lines and a mouse model of the disease.
Epigenetics, the study of chemical modifications that don’t alter the underlying DNA sequence, plays a crucial role in cancer development. These changes can facilitate malignancy by modulating gene expression without any genetic mutations. In cancer biology, DNA methylation abnormalities are known to be a driving force behind tumor growth and metastasis, raising the urgent need for innovative therapeutic strategies that target these epigenetic alterations.
Stephen Baylin, M.D., a co-author of the study and a prominent figure in oncology and medicine at Johns Hopkins, emphasizes the critical need for novel therapeutic options in the fight against solid tumors, which represent a significant share of cancer-related mortality. He points to the unmet demand for treatments that can effectively block aberrant DNA methylation in cancerous cells, underscoring the importance of this research in addressing an ongoing challenge in oncology.
The research methodology specifically targeted UHRF1, a key epigenetic protein that has been identified as a prevalent factor in many solid tumors. Acting as a recruitment factor for other proteins to add methyl groups to DNA, UHRF1 is a logical target in the quest to hinder cancer’s progress. Understanding the role of STELLA in sequestering UHRF1, the research team conducted a thorough investigation into how this interaction might be utilized to mitigate epigenetic-driven oncology.
The discovery that mSTELLA could bind tightly to UHRF1, unlike its hSTELLA counterpart, sheds light on the molecular mechanisms at play. The proteins exhibit only a 31% similarity in their sequences at the amino acid level, indicating that even small differences in protein structure can lead to significant variations in function. This premise leads to an important question: Can the superior binding properties of mouse STELLA be translated into effective treatment for human cancer cells?
The researchers harnessed these insights to explore the creation of a drug regimen featuring the mSTELLA peptide. Testing confirmed that introducing this peptide into human colorectal cancer cells successfully inhibited UHRF1 activity, resulting in the activation of tumor suppressor genes — a crucial factor in the fight against malignant transformation. This innovative application demonstrates a valuable intersection of molecular biology and therapeutic development that could pave the way for new treatment protocols.
A significant breakthrough in this study was the formulation of a lipid nanoparticle therapy to deliver mSTELLA, employed similarly to how mRNA vaccines operate. This drug delivery mechanism proved effective in activating tumor suppressor pathways within cancer cells, significantly impairing tumor growth in mice. Such strategies may represent a new frontier in cancer treatment, especially for solid tumors which, until now, have been challenging to address through epigenetic approaches.
With UHRF1 recognized as an oncogene in various cancers, these findings could hold substantial applicability across multiple types of malignancies. Both Stephen Baylin and Xiangqian Kong, another principal investigator involved in the study, expressed their optimism about translating these results into clinical settings for patients battling cancer. Their excitement underscores the urgency and potential of this research to propel forward the field of epigenetic therapy in oncology, offering hope amidst the current limitations faced in treating solid tumors.
As researchers continue to elucidate the mechanisms underlying STELLA’s interactions and its role in the broader context of cancer biology, the implications of this work could extend beyond colorectal cancer. The potential to rectify epigenetic changes through such targeted therapies presents a formidable tool in the landscape of future cancer treatments, which could ultimately enhance patient outcomes and survival rates.
In addition to establishing a foundation for mSTELLA-based therapies, this research contributes to the larger conversation surrounding the relevance of epigenetic modifications in human health. By bridging molecular insights with practical applications, scientists are making strides toward redefining therapeutic paradigms in oncology, validating the immense promise of epigenetic research in combating cancers that traditionally resist current treatment methodologies.
As the investigation progresses, with support from prominent research grants and collaborations, the revelations from this study could serve as a catalyst for subsequent research initiatives. The scientific community is poised to monitor the developments stemming from this work closely. More importantly, the anticipation surrounding these findings offers a reminder of the relentless pursuit of innovation in medicine — a pursuit that continues to seek solutions for the complex challenges posed by cancer.
Subject of Research: Epigenetic Mechanisms in Colorectal Cancer Treatment
Article Title: Epigenetic Research Offers Hope for Colorectal Cancer Treatment
News Publication Date: January 8, 2024
Web References: https://www.nature.com/articles/s41467-024-55481-7
References: Not Available
Image Credits: Credit: Elizabeth Cook
Keywords: Colorectal cancer, DNA binding proteins, mutant proteins, cancer research, chemical reactions, cell therapies, solid tumors, genomic DNA, gene therapy