Credit: National Human Genome Research Institute.
Infants born prematurely may require parenteral or intravenous nutrition to provide the necessary nourishment, as their digestive system is immature and cannot digest nutrients. However, prolonged parenteral nutrition is associated with complications, including cholestasis, or lack of bile flow from the liver into the small intestine, which leads to accumulation of bile acids and injury in the liver. Emerging clinical studies have shown that cholestasis can be prevented in preterm infants with parenteral administration of oil emulsions ? mixtures made of multiple oil components ? but the mechanism mediating this effect remains unclear.
Working with preterm piglets, an international group led by researchers at the USDA-ARS Children’s Nutrition Research Center (CNRC) at Baylor College of Medicine and Texas Children’s Hospital found evidence that the protective effect of parenteral oil infusions is accompanied by changes in the levels of gut bile acids (gut bile acid pools) and in the gut microbiome, making this study the first to connect parenteral oil infusions, the microbiome, metabolism and health. The study appears in the Journal of Lipid Research.
Studying parenteral oil emulsions
“The piglet model enables us to study parenteral nutrition-associated liver diseases, such as cholestasis, in a way that is clinically relevant,” said senior author Dr. Douglas Burrin, research physiologist at the CNRC and professor of pediatrics at Baylor. “We treat preterm piglets similarly to how we treat preterm infants in the hospital and look at liver function and gene expression in the piglets to better understand the physiology.”
The original lipid emulsion developed for parenteral nutrition in infants was based on one component, soybean oil, and it has been the only parenteral lipid option used for preterm infants for about 45 years. Although this oil emulsion has helped support infants’ growth, physicians have been concerned that it could be involved in the development of several conditions, including liver disease. This prompted the development of new lipid emulsions with multiple oil components to prevent or treat parenteral nutrition-associated liver diseases.
When the first fish-oil and multicomponent lipid emulsions became available, Burrin and his colleagues were the first group to examine their metabolic effects in parenterally fed, preterm piglets. They published their first findings in 2014. The Baylor researchers and others have shown that pure fish oil and multicomponent oil lipid formulations can reduce cholestasis associated with long-term parenteral nutrition, but how this happens still is not completely understood.
In the current study, the researchers expanded their original investigations by comparing two previously studied oil emulsions ? soybean oil only (Intralipid) and a combination of soy, olive, coconut and fish oils (SMOFlipid) ? and a new experimental formulation (EXP), that was similar to SMOFlipid, but with additional DHA, an omega-3 fatty acid, and arachidonic acid.
An additional experimental group (ENT) used for reference consisted of piglets fed infant formula through a feeding tube. The experiment lasted 22 days.
New insights into how parenteral oil emulsions work
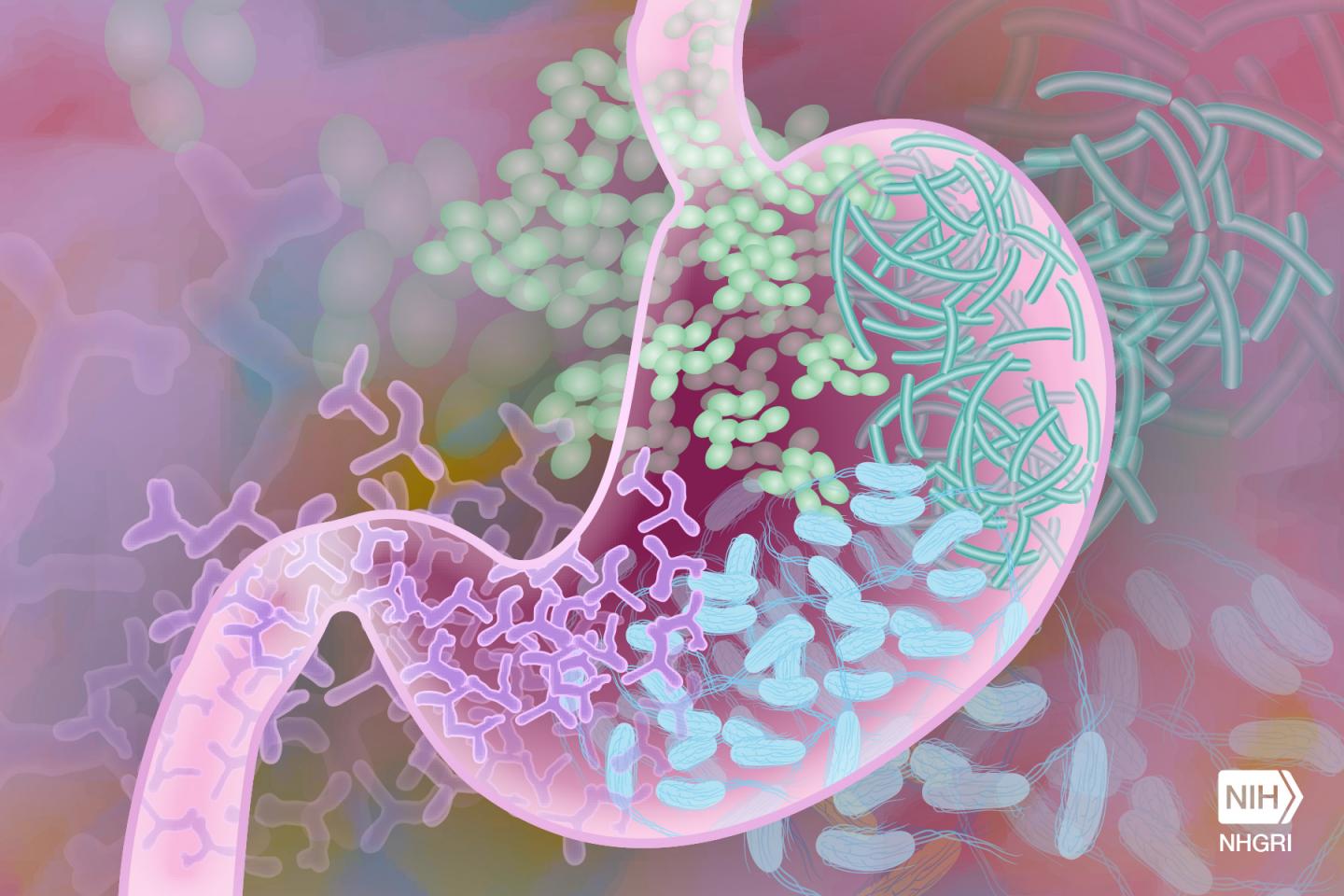
The researchers evaluated the effects of the different oil emulsions in preterm piglets by measuring cholestasis, gut bile acids pools and the composition of microbial communities in the colon as well as the profiles of the microbes’ metabolic products or metabolites.
The findings confirmed that multicomponent oil emulsions (SMOF and EXP), but not Intralipid, can prevent cholestasis and restore bile flow in preterm piglets as observed in the ENT group.
“One of the important findings showed that prevention of cholestasis was accompanied by maintaining normal gut bile acid pools. They were lowest in the piglets treated with Intralipid but increased in the SMOF and EXP groups and were comparable to ENT,” Burrin said.
A particularly interesting new finding was that cholestasis was associated with changes in the gut microbiome and their metabolite profile.
“It’s exciting to see such a direct connection between gut bacteria and the lipid composition of parenteral nutrition,” said first author Dr. Lee Call, a former Translational Biology and Molecular Medicine graduate student in the Burrin lab during the development of this work. Call currently is a postdoctoral fellow at Department of Energy Joint Genome Institute at Lawrence Berkeley National Lab.
“At first it may not seem likely that intravenous lipids could have a large effect on bacterial growth in the intestine, but in fact we see that there is a strong correlation between the type of lipids given parenterally and the relative abundance of certain groups of gut bacteria. And it seems that bile is the connecting link,” Call said. “These results help us understand more about the effects of parenteral nutrition, which is often a life-saving treatment for preterm babies.”
“We have been certain that the lipid emulsions contribute an important effect on growth and metabolism, but the mechanism and the direct causal effect was lacking. This work provides those missing links that offer newer insights that will go a long way in the development of better, safer lipid emulsions for use in preterm infants. It is exciting to be a part of this discovery,” said co-author Dr. Muralidhar Premkumar, assistant professor of pediatrics-newborn at Baylor and Texas Children’s.
###
Other contributors to this work include Tiffany Molina, Barbara Stoll, Greg Guthrie, Shaji Chacko, Jogchum Plat, Jason Robinson, Sen Lin, Caitlin Vonderohe, Mahmoud Mohammad, Dennis Kunichoff, Stephanie Cruz, Patricio Lau, Jon Nielsen, Zhengfeng Fang, Oluyinka Olutoye, Thomas Thymann, Robert Britton and Per Sangild. The authors are affiliated with one of more of the following institutions: Baylor College of Medicine; USDA-ARS Children’s Nutrition Research Center; Maastricht University, Netherlands; Sichuan Agricultural University, China; University of Copenhagen, Denmark.
This work was supported by federal funds from the USDA, Agricultural Research Service under Cooperative Agreement Number 3092-51000-060-01, grants from the University of Copenhagen, Fresenius Kabi, the Whitlock Foundation, National Institutes of Health (Grants DK-094616 and T32-GM088129), and the Texas Medical Center Digestive Diseases Center (NIH Grant P30 DK-56338). Further support was provided by the Gulf Coast Consortia and an NLM Training Program in Biomedical Informatics (T15-LM007093).
Media Contact
Homa Shalchi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.